Abstract
Background
M2-like macrophages are associated with the pathogenesis of castrate-resistant prostate cancer (CRPC). We sought to determine if dietary omega-3 fatty acids (ω-3 FAs) delay the development and progression of CRPC and inhibit tumor-associated M2-like macrophages.
Methods
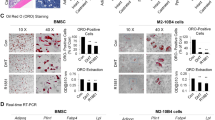
MycCap cells were grown subcutaneously in immunocompetent FVB mice. Mice were castrated when tumors reached 300 mm2. To study effects of dietary ω-3 FAs on development of CRPC, ω-3 or ω-6 diets were started 2 days after castration and mice sacrificed after early regrowth of tumors. To study ω-3 FA effects on progression of CRPC, tumors were allowed to regrow after castration before starting the diets. M2 (CD206+) macrophages were isolated from allografts to examine ω-3 FA effects on macrophage function. Omega-3 fatty acid effects on androgen-deprived RAW264.7 M2 macrophages were studied by RT-qPCR and a migration/ invasion assay.
Results
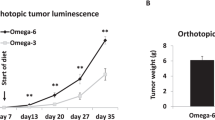
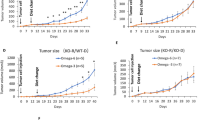
The ω-3 diet combined with castration lead to greater MycCap tumor regression (tumor volume reduction: 182.2 ± 33.6 mm3) than the ω-6 diet (tumor volume reduction: 148.3 ± 35.2; p = 0.003) and significantly delayed the time to CRPC (p = 0.006). Likewise, the ω-3 diet significantly delayed progression of established castrate-resistant MycCaP tumors (p = 0.003). The ω-3 diet (as compared to the ω-6 diet) significantly reduced tumor-associated M2-like macrophage expression of CSF-1R in the CRPC development model, and matrix metallopeptidase-9 (MMP-9) and vascular endothelial growth factor (VEGF) in the CRPC progression model. Migration of androgen-depleted RAW264.7 M2 macrophages towards MycCaP cells was reversed by addition of docosahexaenoic acid (ω-3).
Conclusions
Dietary omega-3 FAs (as compared to omega-6 FAs) decreased the development and progression of CRPC in an immunocompetent mouse model, and had inhibitory effects on M2-like macrophage function. Clinical trials are warranted evaluating if a fish oil-based diet can delay the time to castration resistance in men on androgen deprivation therapy, whereas further preclinical studies are warranted evaluating fish oil for more advanced CRPC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Augustsson K, Michaud DS, Rimm EB, Leitzmann MF, Stampfer MJ, Willett WC, et al. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:64–67.
Brasky TM, Darke AK, Song X, Tangen CM, Goodman PJ, Thompson IM, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2013;105:1132–41.
Brasky TM, Till C, White E, Neuhouser ML, Song X, Goodman P, et al. Serum phospholipid fatty acids and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Am J Epidemiol. 2011;173:1429–39.
Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–16.
Lovegrove C, Ahmed K, Challacombe B, Khan MS, Popert R, Dasgupta P. Systematic review of prostate cancer risk and association with consumption of fish and fish-oils: analysis of 495,321 participants. Int J Clin Pract. 2015;69:87–105.
Norrish AE, Skeaff CM, Arribas GL, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Br J Cancer. 1999;81:1238–42.
Friedrichs W, Ruparel SB, Marciniak RA, deGraffenried L. Omega-3 fatty acid inhibition of prostate cancer progression to hormone independence is associated with suppression of mTOR signaling and androgen receptor expression. Nutr Cancer. 2011;63:771–7.
Lloyd JC, Masko EM, Wu C, Keenan MM, Pilla DM, Aronson WJ, et al. Fish oil slows prostate cancer xenograft growth relative to other dietary fats and is associated with decreased mitochondrial and insulin pathway gene expression. Prostate Cancer Prostatic Dis. 2013;16:285–91.
Wang S, Wu J, Suburu J, Gu Z, Cai J, Axanova LS, et al. Effect of dietary polyunsaturated fatty acids on castration-resistant Pten-null prostate cancer. Carcinogenesis. 2012;33:404–12.
Gevariya N, Besancon M, Robitaille K, Picard V, Diabate L, Alesawi A, et al. Omega-3 fatty acids decrease prostate cancer progression associated with an anti-tumor immune response in eugonadal and castrated mice. Prostate. 2019;79:9–20.
Liang P, Henning SM, Schokrpur S, Wu L, Doan N, Said J, et al. Effect of ietary omega-3 fatty acids on tumor-associated macrophages and prostate cancer progression. Prostate. 2016;76:1293–302.
Lundholm M, Hagglof C, Wikberg ML, Stattin P, Egevad L, Bergh A, et al. Secreted factors from colorectal and prostate cancer cells skew the immune response in opposite directions. Sci Rep. 2015;5:15651.
Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2014;33:2423–31.
Lanciotti M, Masieri L, Raspollini MR, Minervini A, Mari A, Comito G, et al. The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. BioMed Res Int. 2014;2014:486798.
Zarif JC, Taichman RS, Pienta KJ. TAM macrophages promote growth and metastasis within the cancer ecosystem. Oncoimmunology. 2014;3:e941734.
Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460.
Silva JAF, Bruni-Cardoso A, Augusto TM, Damas-Souza DM, Barbosa GO, Felisbino SL, et al. Macrophage roles in the clearance of apoptotic cells and control of inflammation in the prostate gland after castration. Prostate. 2018;78:95–103.
Escamilla J, Schokrpur S, Liu C, Priceman SJ, Moughon D, Jiang Z, et al. CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res. 2015;75:950–62.
Zarif JC, Yang W, Hernandez JR, Zhang H, Pienta KJ. The identification of macrophage-enriched glycoproteins using glycoproteomics. Mol Cell Proteom. 2017;16:1029–37.
Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–38.
Watson CS, Bialek P, Anzo M, Khosravi J, Yee SP, Han VK. Elevated circulating insulin-like growth factor binding protein-1 is sufficient to cause fetal growth restriction. Endocrinology. 2006;147:1175–86.
Zhang C, Wang Y, Wang F, Wang Z, Lu Y, Xu Y, et al. Quantitative profiling of glycerophospholipids during mouse and human macrophage differentiation using targeted mass spectrometry. Sci Rep. 2017;7:412.
Liang P, Henning SM, Guan J, Grogan T, Elashoff D, Olefsky JM, et al. Role of host GPR120 in mediating dietary omega-3 fatty acid inhibition of prostate cancer. J Natl Cancer Inst. 2019;111:52–59.
Ngo TH, Barnard RJ, Anton T, Tran C, Elashoff D, Heber D, et al. Effect of isocaloric low-fat diet on prostate cancer xenograft progression to androgen independence. Cancer Res. 2004;64:1252–4.
Ngo TH, Barnard RJ, Cohen P, Freedland S, Tran C, deGregorio F, et al. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin Cancer Res. 2003;9:2734–43.
Mateo J, Fizazi K, Gillessen S, Heidenreich A, Perez-Lopez R, Oyen WJG, et al. Managing nonmetastatic castration-resistant prostate cancer. Eur Urol. 2019;75:285–93.
Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–5.
Lin TH, Izumi K, Lee SO, Lin WJ, Yeh S, Chang C. Anti-androgen receptor ASC-J9 versus anti-androgens MDV3100 (Enzalutamide) or Casodex (Bicalutamide) leads to opposite effects on prostate cancer metastasis via differential modulation of macrophage infiltration and STAT3-CCL2 signaling. Cell Death Dis. 2013;4:e764.
Ren X, Fu X, Zhang X, Chen S, Huang S, Yao L, et al. Testosterone regulates 3T3-L1 pre-adipocyte differentiation and epididymal fat accumulation in mice through modulating macrophage polarization. Biochem Pharmacol. 2017;140:73–88.
Zhang P, Zhao S, Wu C, Li J, Li Z, Wen C, et al. Effects of CSF1R-targeted chimeric antigen receptor-modified NK92MI & T cells on tumor-associated macrophages. Immunotherapy. 2018;10:935–49.
Berquin IM, Edwards IJ, Kridel SJ, Chen YQ. Polyunsaturated fatty acid metabolism in prostate cancer. Cancer Metast Rev. 2011;30:295–309.
Aucoin M, Cooley K, Knee C, Fritz H, Balneaves LG, Breau R, et al. Fish-derived omega-3 fatty acids and prostate cancer: a systematic review. Integr Cancer Ther. 2017;16:32–62.
Gu Z, Wu J, Wang S, Suburu J, Chen H, Thomas MJ, et al. Polyunsaturated fatty acids affect the localization and signaling of PIP3/AKT in prostate cancer cells. Carcinogenesis. 2013;34:1968–75.
Acknowledgements
We thank Howard B. Klein for his generous support.
Funding
This work was supported by the National Institute of Health (P50CA92131 to WJA; RO1CA231219 to WJA and PC and P30CA016042 and used the flow cytometry core of the CCSG shared resource) and Department of Defense Prostate Cancer Research Program (PC141593 to PL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liang, P., Henning, S.M., Guan, J. et al. Effect of dietary omega-3 fatty acids on castrate-resistant prostate cancer and tumor-associated macrophages. Prostate Cancer Prostatic Dis 23, 127–135 (2020). https://doi.org/10.1038/s41391-019-0168-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-019-0168-8
This article is cited by
-
Impact of serum eicosapentaenoic acid/arachidonic acid ratio on overall survival in lung cancer patients treated with pembrolizumab: a pilot study
Scientific Reports (2024)
-
Effect of omega-3 fatty acid diet on prostate cancer progression and cholesterol efflux in tumor-associated macrophages—dependence on GPR120
Prostate Cancer and Prostatic Diseases (2023)
-
SNAP25 is a potential prognostic biomarker for prostate cancer
Cancer Cell International (2022)
-
Effects of dietary omega-3 fatty acids on orthotopic prostate cancer progression, tumor associated macrophages, angiogenesis and T-cell activation—dependence on GPR120
Prostate Cancer and Prostatic Diseases (2022)