Abstract
Background
Over the past decade prostate cancer (PCa) diagnostic approaches have evolved away from aggressive prostate-specific antigen (PSA) screening. While a goal of these changes is to decrease over diagnosis and treatment, little is known about the downstream effects on PCa risk distribution at the time of diagnosis. To better understand these effects, we used a national cohort of men to investigate temporal trends in PCa risk profile at diagnosis.
Methods
Using the National Cancer Database, we identified men diagnosed with biopsy-confirmed clinically localized prostate adenocarcinoma (T1-4N0M0) from 2004 to 2014. We assessed temporal trends in proportional distribution of National Comprehensive Cancer Network risk groups as well as their sub-components (PSA, Gleason score, clinical T stage). We also evaluated trends in these sub-components among men with intermediate- and high-risk disease as well as those with metastatic disease.
Results
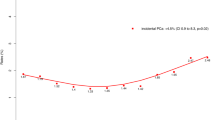
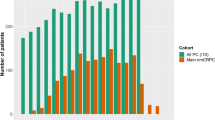
In our cohort of 755,567 men diagnosed between 2004 and 2014, there was a decrease in the proportion of men diagnosed with low-risk PCa (38.32 to 27.23%, p < 0.001) and a consequent increase in the proportion of localized intermediate-risk (40.49 to 46.72%, p < 0.001) and high-risk diagnoses (21.19 to 26.05%, p < 0.001). This was primarily driven by an increased proportion of Gleason 7 and Gleason 8–10 cancer, respectively. The number of men presenting with metastatic disease consistently increased from 3251 (2.88%) in 2004 to 6886 (7.19%) in 2014 (p < 0.001).
Conclusions
The proportion of localized intermediate/high risk and metastatic PCa has substantially increased over the past decade, while the proportion of low-risk disease has decreased. This shift has been primarily driven by increased diagnosis of high-grade disease. National guidelines advising against PSA screening may have contributed to these findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cooperberg MR, Lubeck DP, Mehta SS, Carroll PR, CaPsure. Time trends in clinical risk stratification for prostate cancer: implications for outcomes (data from CaPSURE). J Urol. 2003;170(Pt 2):S21–5. discussion S26–7.
Shao YH, Demissie K, Shih W, Mehta AR, Stein MN, Roberts CB, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101:1280–3.
Moyer VA, Force USPST. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–34.
Force USPST. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185–91.
Jemal A, Fedewa SA, Ma J, Siegel R, Lin CC, Brawley O, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314:2054–61.
Sammon JD, Abdollah F, Choueiri TK, Kantoff PW, Nguyen PL, Menon M, et al. Prostate-specific antigen screening after 2012 US preventive services task force recommendations. JAMA. 2015;314:2077–9.
Negoita S, Feuer EJ, Mariotto A, Cronin KA, Petkov VI, Hussey SK et al. Annual Report to the Nation on the Status of Cancer, Part II: Recent changes in prostate cancer trends and disease characteristics. Cancer 2018;124:2801–14.
Lerro CC, Robbins AS, Phillips JL, Stewart AK. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20:1759–65.
Epstein JI, Allsbrook WC Jr., Amin MB, Egevad LL, Committee IG. The 2005 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–42.
Chambers R, Skinney CJ. Analysis of survey data. John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, England; 2003.
Ohmann EL, Loeb S, Robinson D, Bill-Axelson A, Berglund A, Stattin P. Nationwide, population-based study of prostate cancer stage migration between and within clinical risk categories. Scand J Urol. 2014;48:426–35.
Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, Gann PH, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–90.
Hu JC, Nguyen P, Mao J, Halpern J, Shoag J, Wright JD, et al. Increase in prostate cancer distant metastases at diagnosis in the United States. JAMA Oncol. 2017;3:705–7.
Shariat SF, Kattan MW, Vickers AJ, Karakiewicz PI, Scardino PT. Critical review of prostate cancer predictive tools. Future Oncol. 2009;5:1555–84.
Caras RJ, Sterbis JR. Prostate cancer nomograms: a review of their use in cancer detection and treatment. Curr Urol Rep. 2014;15:391.
Greene KL, Meng MV, Elkin EP, Cooperberg MR, Pasta DJ, Kattan MW, et al. Validation of the Kattan preoperative nomogram for prostate cancer recurrence using a community based cohort: results from cancer of the prostate strategic urological research endeavor (capsure). J Urol. 2004;171(Pt 1):2255–9.
Steyerberg EW, Roobol MJ, Kattan MW, van der Kwast TH, de Koning HJ, Schroder FH. Prediction of indolent prostate cancer: validation and updating of a prognostic nomogram. J Urol. 2007;177:107–12. discussion 112
Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–42.
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24.
Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13.
Albertsen PC, Hanley JA, Barrows GH, Penson DF, Kowalczyk PD, Sanders MM, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248–53.
Kondylis FI, Moriarty RP, Bostwick D, Schellhammer PF. Prostate cancer grade assignment: the effect of chronological, interpretive and translation bias. J Urol. 2003;170(Pt 1):1189–93.
Weiner AB, Patel SG, Etzioni R, Eggener SE. National trends in the management of low and intermediate risk prostate cancer in the United States. J Urol. 2015;193:95–102.
Reese AC, Wessel SR, Fisher SG, Mydlo JH. Evidence of prostate cancer “reverse stage migration” toward more advanced disease at diagnosis: data from the Pennsylvania Cancer Registry. Urol Oncol. 2016;34:335 e321–338.
Chen N, Zhou Q. The evolving Gleason grading system. Chin J Cancer Res. 2016;28:58–64.
Danneman D, Drevin L, Robinson D, Stattin P, Egevad L. Gleason inflation 1998–2011: a registry study of 97,168 men. BJU Int. 2015;115:248–55.
Sebo TJ, Bock BJ, Cheville JC, Lohse C, Wollan P, Zincke H. The percent of cores positive for cancer in prostate needle biopsy specimens is strongly predictive of tumor stage and volume at radical prostatectomy. J Urol. 2000;163:174–8.
Freedland SJ, Csathy GS, Dorey F, Aronson WJ. Percent prostate needle biopsy tissue with cancer is more predictive of biochemical failure or adverse pathology after radical prostatectomy than prostate specific antigen or Gleason score. J Urol. 2002;167(Pt 1):516–20.
D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Fondurulia J, Chen MH, et al. Clinical utility of the percentage of positive prostate biopsies in defining biochemical outcome after radical prostatectomy for patients with clinically localized prostate cancer. J Clin Oncol. 2000;18:1164–72.
Spalding AC, Daignault S, Sandler HM, Shah RB, Pan CC, Ray ME. Percent positive biopsy cores as a prognostic factor for prostate cancer treated with external beam radiation. Urology. 2007;69:936–40.
Acknowledgements
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Funding
ASK is supported by the DiNovi Family Fund. QDT is supported by an unrestricted educational grant from the Vattikuti Urology Institute, a Clay Hamlin Young Investigator Award from the Prostate Cancer Foundation and a Genentech BioOncology Career Development Award from the Conquer Cancer Foundation of the American Society of Clinical Oncology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ASK reports consulting fees from, Insightec, Merck, Pfizer, Janssen, and Profound Medical. QDT reports honoraria from Bayer and Astellas US. The remaing authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fletcher, S.A., von Landenberg, N., Cole, A.P. et al. Contemporary national trends in prostate cancer risk profile at diagnosis. Prostate Cancer Prostatic Dis 23, 81–87 (2020). https://doi.org/10.1038/s41391-019-0157-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-019-0157-y
This article is cited by
-
A DNA copy number alteration classifier as a prognostic tool for prostate cancer patients
British Journal of Cancer (2023)
-
Loss of PDE4D7 expression promotes androgen independence, neuroendocrine differentiation and alterations in DNA repair: implications for therapeutic strategies
British Journal of Cancer (2023)
-
The impact of race/ethnicity on upstaging and/or upgrading rates among intermediate risk prostate cancer patients treated with radical prostatectomy
World Journal of Urology (2022)
-
Onset and burden of lower limb lymphedema after radical prostatectomy: a cross-sectional study
Supportive Care in Cancer (2022)
-
Development and validation of a preoperative nomogram for predicting survival of patients with locally advanced prostate cancer after radical prostatectomy
BMC Cancer (2020)