Abstract
Background
Tumor contact length (TCL) is defined as the extent of contact between prostate cancer and the prostatic capsule, and its predictive value for microscopic extraprostatic extension (EPE) has been reported. However, the impact of the zonal origin (anterior or posterior tumor) of the tumor on the diagnosis of EPE is controversial.
Methods
We retrospectively analyzed the records of 233 consecutive patients who underwent preoperative MRI and radical prostatectomy. We designated their tumors as anterior or posterior, and evaluated the correlation between the TCL measured by MRI and microscopic EPE in the radical prostatectomy specimen. Then, we created the predicted probability curves for EPE versus TCL for anterior and posterior prostate cancer.
Results
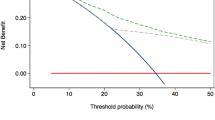
There were 109 patients (47%) with an anterior tumor and 124 patients (53%) with a posterior tumor. Postoperative pathological analysis confirmed pT3 in 18 patients (17%) with an anterior tumor and in 53 patients (43%) with a posterior tumor. Multivariate analysis demonstrated that the zonal origin of the tumor was an independent predictive factor for EPE. We developed separate probability curves of EPE versus TCL for anterior and posterior prostate cancer, which revealed that anterior tumors were less likely to invade the extraprostatic tissues. Among patients whose TCL was 10–20 mm, 9/32 patients (28%) with an anterior tumor had EPE compared with 24/45 patients (53%) with a posterior tumor (p = 0.036). The decision curve of this EPE predictive model had high clinical efficacy.
Conclusions
Our results indicate that anterior tumors have more favorable pathological characteristics than posterior tumors with the same TCL measured by MRI. We constructed two separate predicted probability curves for EPE after discriminating anterior and posterior tumors, which will be useful for decision making in clinical practice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ball MW, Partin AW, Epstein JI. Extent of extraprostatic extension independently influences biochemical recurrence-free survival: evidence for further pT3 subclassification. Urology. 2015;85:161–4.
Satake N, Ohori M, Yu C, Kattan MW, Ohno Y, Miyakawa A, et al. Development and internal validation of a nomogram predicting extracapsular extension in radical prostatectomy specimens. Int J Urol. 2010;17:267–72.
Tosoian JJ, Chappidi M, Feng Z, Humphreys EB, Han M, Pavlovich CP, et al. Prediction of pathological stage based on clinical stage, serum prostate-specific antigen, and biopsy Gleason score: Partin Tables in the contemporary era. BJU Int. 2017;119:676–83.
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69:16–40.
Ueno Y, Tamada T, Bist V, Reinhold C, Miyake H, Tanaka U, et al. Multiparametric magnetic resonance imaging: current role in prostate cancer management. Int J Urol. 2016;23:550–7.
Boesen L, Chabanova E, Løgager V, Balslev I, Mikines K, Thomsen HS. Prostate cancer staging with extracapsular extension risk scoring using multiparametric MRI: a correlation with histopathology. Eur Radiol. 2015;25:1776–85.
Baco E, Rud E, Vlatkovic L, Svindland A, Eggesbø HB, Hung AJ, et al. Predictive value of magnetic resonance imaging determined tumor contact length for extracapsular extension of prostate cancer. J Urol. 2015;193:466–72.
Rosenkrantz AB, Shanbhogue AK, Wang A, Kong MX, Babb JS, Taneja SS. Length of capsular contact for diagnosing extraprostatic extension on prostate MRI: assessment at an optimal threshold. J Magn Reson Imaging. 2016;43:990–7.
Woo S, Kim SY, Cho JY, Kim SH. Length of capsular contact on prostate MRI as a predictor of extracapsular extension: which is the most optimal sequence? Acta Radiol. 2017;58:489–97.
Kongnyuy M, Sidana A, George AK, Muthigi A, Iyer A, Ho R, et al. Tumor contact with prostate capsule on magnetic resonance imaging: a potential biomarker for staging and prognosis. Urol Oncol. 2017;35:30.e1–8.
Costa DN, Passoni NM, Leyendecker JR, de Leon AD, Lotan Y, Roehrborn CG. Diagnostic utility of a Likert scale versus qualitative descriptors and length of capsular contact for determining extraprostatic tumor extension at multiparametric prostate MRI. AJR Am J Roentgenol. 2018;210:1066–72.
Rud E, Diep L, Baco E. A prospective study evaluating indirect MRI-signs for the prediction of extraprostatic disease in patients with prostate cancer: tumor volume, tumor contact length and tumor apparent diffusion coefficient. World J Urol. 2018;36:629–37.
Takahashi H, Epstein JI, Wakui S, Yamamoto T, Furusato B, Zhang M. Differences in prostate cancer grade, stage, and location in radical prostatectomy specimens from United States and Japan. Prostate. 2014;74:321–5.
Sato S, Takahashi H, Kimura T, Egawa S, Furusato B, Ikegami M. Clinicopathological importance of anterior prostate cancer in Japanese Men. Pathol Int. 2017;67:156–62.
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA; Grading Committee. International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–52.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak. 2006;26:565–74.
Al-Ahmadie HA, Tickoo SK, Olgac S, Gopalan A, Scardino PT, Reuter VE, et al. Anterior-predominant prostatic tumors: zone of origin and pathologic outcomes at radical prostatectomy. Am J Surg Pathol. 2008;32:229–35.
Ukimura O, Troncoso P, Ramirez EI, Babaian RJ. Prostate cancer staging: correlation between ultrasound determined tumor contact length and pathologically confirmed extraprostatic extension. J Urol. 1998;159:1251–9.
Rud E, Klotz D, Rennesund K, Baco E, Berge V, Lien D, et al. Detection of the index tumour and tumour volume in prostate cancer using T2-weighted and diffusion-weighted magnetic resonance imaging (MRI) alone. BJU Int. 2014;114:E32–42.
Sundi D, Kryvenko ON, Carter HB, Ross AE, Epstein JI, Schaeffer EM. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black American men. J Urol. 2014;191:60–7.
Shaikhibrahim Z, Lindstrot A, Ellinger J, Rogenhofer S, Buettner R, Perner S, et al. The peripheral zone of the prostate is more prone to tumor development than the transitional zone: is the ETS family the key? Mol Med Rep. 2012;5:313–6.
Guo CC, Zuo G, Cao D, Troncoso P, Czerniak BA. Prostate cancer of transition zone origin lacks TMPRSS2-ERG gene fusion. Mod Pathol. 2009;22:866–71.
Gopalan A, Leversha MA, Dudas ME, Maschino AC, Chang J, Al-Ahmadie HA, et al. TMPRSS2-ERG rearrangement in dominant anterior prostatic tumours: incidence and correlation with ERG immunohistochemistry. Histopathology. 2013;63:279–86.
Leyten GH, Hessels D, Jannink SA, Smit FP, de Jong H, Cornel EB, et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol. 2014;65:534–42.
Fine SW, Al-Ahmadie HA, Gopalan A, Tickoo SK, Scardino PT, Reuter VE. Anatomy of the anterior prostate and extraprostatic space: a contemporary surgical pathology analysis. Adv Anat Pathol. 2007;14:401–7.
Bouyé S, Potiron E, Puech P, Leroy X, Lemaitre L, Villers A. Transition zone and anterior stromal prostate cancers: zone of origin and intraprostatic patterns of spread at histopathology. Prostate. 2009;69:105–13.
Hassan MO, Maksem J. The prostatic perineural space and its relation to tumor spread: an ultrastructural study. Am J Surg Pathol. 1980;4:143–8.
Ohori M, Scardino PT, Lapin SL, Seale-Hawkins C, Link J, Wheeler TM. The mechanisms and prognostic significance of seminal vesicle involvement by prostate cancer. Am J Surg Pathol. 1993;17:1252–61.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsumoto, K., Akita, H., Narita, K. et al. Prediction of extraprostatic extension by MRI tumor contact length: difference between anterior and posterior prostate cancer. Prostate Cancer Prostatic Dis 22, 539–545 (2019). https://doi.org/10.1038/s41391-019-0136-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-019-0136-3
This article is cited by
-
Prostate Cancer and Prostatic Diseases Best of Asia, 2019: challenges and opportunities
Prostate Cancer and Prostatic Diseases (2020)
-
Extraprostatic extension in prostate cancer: primer for radiologists
Abdominal Radiology (2020)