Abstract
Background
Enzalutamide can significantly prolong the survival of patients with metastatic castration-resistant prostate cancer (mCRPC). However, there is a paucity of evidence on continuing androgen-deprivation therapy (ADT) for mCRPC. Here, we analyzed the effect of concomitant ADT during enzalutamide treatment in men with mCRPC following chemotherapy.
Methods
We retrospectively reviewed the medical records of 232 patients with mCRPC who received oral enzalutamide (160 mg per day) following chemotherapy at 9 tertiary centers in Korea between 2014 and 2016. The primary endpoint was overall survival, while secondary endpoints included time to prostate-specific antigen (PSA) progression and radiographic progression-free survival.
Results
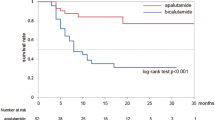
The median age of the patients was 71 years (interquartile range, 64–75 years). The proportion of patients in a grade group ≥4 was 77.6%. The rate of concomitant ADT was 29.3%, and the all-cause mortality rate was 27.1% (n = 63). Median overall survival, time to PSA progression, and radiographic progression-free survival were 24.0, 8.0, and 10.0 months, respectively. Notably, concomitant ADT showed a significant association with longer overall survival (median duration not reached vs. 18.2 months; p = 0.008). After adjusting for confounding factors, concomitant ADT was still associated with longer overall survival (hazard ratio, 0.35; 95% confidence interval, 0.17–0.72).
Conclusion
Concomitant ADT during enzalutamide treatment may improve the survival of patients with mCRPC following chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schalken J, Fitzpatrick JM. Enzalutamide: targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int. 2016;117:215–25.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomark Prev. 2016;25:16–27.
Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92.
Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47:127–41.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Shamash J, Davies A, Ansell W, McFaul S, Wilson P, Oliver T, et al. A phase II study investigating the re-induction of endocrine sensitivity following chemotherapy in androgen-independent prostate cancer. Br J Cancer. 2008;98:22–4.
Taylor CD, Elson P, Trump DL. Importance of continued testicular suppression in hormone-refractory prostate cancer. J Clin Oncol. 1993;11:2167–72.
Hussain M, Wolf M, Marshall E, Crawford ED, Eisenberger M. Effects of continued androgen-deprivation therapy and other prognostic factors on response and survival in phase II chemotherapy trials for hormone-refractory prostate cancer: a Southwest Oncology Group report. J Clin Oncol. 1994;12:1868–75.
Lee JL, Eun Kim J, Ahn JH, Lee DH, Lee J, Kim CS, et al. Role of androgen deprivation treatment in patients with castration-resistant prostate cancer, receiving docetaxel-based chemotherapy. Am J Clin Oncol. 2011;34:140–4.
Bong GW, Clarke HS Jr, Hancock WC, Keane TE. Serum testosterone recovery after cessation of long-term luteinizing hormone-releasing hormone agonist in patients with prostate cancer. Urology. 2008;71:1177–80.
Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54.
Morote J, Orsola A, Planas J, Trilla E, Raventos CX, Cecchini L, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178:1290–5.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–51.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–60.
Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71.
Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22.
Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54.
Rexer H. [Study on the therapy of castration-resistant prostate cancer: Randomized phase II study of abiraterone acetate with LHRH therapy vs. abiraterone acetate without LHRH therapy in patients with progressive chemotherapy-naive castration-resistant prostate cancer (SPARE)—AP 67/11 of the Association of Urogenital Oncology (AUO)]. Urologe A. 2016;55:665–7.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–18.
Kim CS, Theeuwes A, Kwon DD, Choi YD, Chung BH, Lee HM, et al. The PREVAIL trial of enzalutamide in men with chemotherapy-naive, metastatic castration-resistant prostate cancer: post hoc analysis of Korean patients. Investig Clin Urol. 2016;57:174–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jeong, C.W., Kang, M., Il Jung, S. et al. Importance of androgen-deprivation therapy during enzalutamide treatment in men with metastatic castration-resistant prostate cancer following chemotherapy: results from retrospective, multicenter data. Prostate Cancer Prostatic Dis 22, 150–158 (2019). https://doi.org/10.1038/s41391-018-0088-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-018-0088-z
This article is cited by
-
Prostate Cancer and Prostatic Diseases Best of Asia, 2019: challenges and opportunities
Prostate Cancer and Prostatic Diseases (2020)