Abstract
Background
Non-metastatic castration resistant prostate cancer (M0CRPC) is a heterogenous disease state affecting an estimated 100,000 men in the United States. Development of more sensitive modalities for detection of metastasis has altered the landscape of advanced prostate cancer, but M0CRPC has remained a condition that previously lacked FDA-approved treatment. The emerging data on new generation Androgen Receptor (AR) pathway inhibitors should address this gap in the management of such patients.
Methods
We reviewed and summarized the current literature for the definition, diagnosis and treatment of M0CRPC. We highlight the results of recent Phase III trials that show significant impact on the outcomes of M0CRPC.
Results
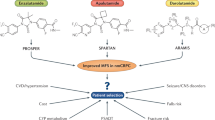
Androgen deprivation therapy remains the foundation of therapy for M0CRPC. Recently published Phase III trials provided data on improved progression free survival when ADT is augmented with newer AR pathway inhibitors. The SPARTAN trial showed that metastasis-free survival (MFS) for patients treated with apalutamide plus ADT is 40.5 months compared to 16.2 months for patients who received standard ADT plus placebo, a 72% reduction in the risk of distant metastasis or death in apalutamide plus ADT compared to ADT plus placebo. The PROSPER trial demonstrated that MFS for patients treated with enzalutamide plus ADT was 36.6 months compared to 14.7 months for patients who received standard ADT only indicating a 71% reduction in the risk of developing metastatic CRPC or death compared to ADT alone. The ARAMIS trial on darolutamide, another AR pathway inhibitor, is also ongoing, and can potentially be another fitting option for M0CRPC.
Conclusion
The recent Phase III trials SPARTAN and PROPSER demonstrate effective treatment options for the M0CRPC disease state that has historically lacked treatment from high level evidence. In particular, an FDA-approved treatment, Apalutamide, can finally be offered for M0CRPC patients. The newer AR pathway inhibitors should provide a basis for further investigation into treatments for M0CRPC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Zappa M, Nelen V, et al. The European Randomized Study of Screening for Prostate Cancer—Prostate Cancer Mortality at 13 Years of Follow-up. Lancet. 2014;384(9959):2027–35.
Draft Recommendation Statement: Prostate Cancer: Screening. 2017.
Trapasso JG, Dekernion JB, Smith RB, Dorey F. The incidence and significance of detectable levels of serum prostate specific antigen after radical prostatectomy. J Urol. 1994;152(5):1821–5.
Roberts SG, Blute ML, Bergstralh EJ, Slezak JM, Zincke H, editors. PSA doubling time as a predictor of clinical progression after biochemical failure following radical prostatectomy for prostate cancer. Mayo Clinic Proceedings; 2001: Elsevier.
Tombal B, Miller K, Boccon-Gibod L, Schröder F, Shore N, Crawford ED, et al. Additional analysis of the secondary end point of biochemical recurrence rate in a phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics. Eur Urol. 2010;57(5):836–42.
SEER Cancer Stat Facts: Prostate Cancer.
Karantanos T, Evans CP, Tombal B, Thompson TC, Montironi R, Isaacs WB. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol. 2015;67(3):470–9.
Afshar M, Evison F, James ND, Patel P, editors. Shifting paradigms in the estimation of survival for castration-resistant prostate cancer: A tertiary academic center experience. Urologic Oncology: Seminars and Original Investigations; 2015: Elsevier.
Chandrasekar T, Yang JC, Gao AC, Evans CP. Targeting molecular resistance in castration-resistant prostate cancer. BMC Med. 2015;13(1):206.
Chandrasekar T, Yang JC, Gao AC, Evans CP. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl Androl Urol. 2015;4(3):365.
Mohler JL, Gregory CW, Ford OH, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10(2):440–8.
Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–54.
Macomson B, Lin JH, Tunceli O, Behl AS, Pericone C, Deshmukh S, et al. Time to metastasis or death in non-metastatic castrate resistant prostate cancer (nmCRPC) patients by National Comprehensive Cancer Network (NCCN) risk groups. American Society of Clinical Oncology; 2017;35:15_suppl, 5027–27.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34(12):1402–18.
Smith MR, Saad F, Oudard S, Shore N, Fizazi K, Sieber P, et al. Denosumab and Bone Metastasis–Free Survival in Men With Nonmetastatic Castration-Resistant Prostate Cancer: Exploratory Analyses by Baseline Prostate-Specific Antigen Doubling Time. J Clin Oncol. 2013;31(30):3800–6.
Scher HI, Solo K, Valant J, Todd MB, Mehra M. Prevalence of prostate cancer clinical states and mortality in the United States: estimates using a dynamic progression model. PLoS ONE. 2015;10(10):e0139440.
Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918–25.
Crawford ED, Stone NN, Evan YY, Koo PJ, Freedland SJ, Slovin SF, et al. Challenges and recommendations for early identification of metastatic disease in prostate cancer. Urology. 2014;83(3):664–9.
Sartor AO, Tangen CM, Hussain MH, Eisenberger MA, Parab M, Fontana JA, et al. Antiandrogen withdrawal in castrate‐refractory prostate cancer. Cancer . 2008;112(11):2393–400.
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56(5):668–74.
Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56(8):1185–90.
Mosavi F, Johansson S, Sandberg DT, Turesson I, Sörensen J, Ahlström H. Whole-body diffusion-weighted MRI compared with 18F-NaF PET/CT for detection of bone metastases in patients with high-risk prostate carcinoma. AJR Am J Roentgenol. 2012;199(5):1114–20.
Umbehr MH, Müntener M, Hany T, Sulser T, Bachmann LM. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013;64(1):106–17.
Geynisman DM, Plimack ER, Zibelman M. Second-generation Androgen Receptor–targeted Therapies in Nonmetastatic Castration-resistant Prostate Cancer: Effective Early Intervention or Intervening Too Early? Eur Urol. 2016;70(6):971–3.
Taylor C, Elson P, Trump D. Importance of continued testicular suppression in hormone-refractory prostate cancer. J Clin Oncol. 1993;11(11):2167–72.
Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253–61.
Group PCTC. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355(9214):1491–8.
Morote J, Orsola A, Planas J, Trilla E, Raventós CX, Cecchini L, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178(4):1290–5.
Lawrentschuk N, Fernandes K, Bell D, Barkin J, Fleshner N. Efficacy of a second line luteinizing hormone-releasing hormone agonist after advanced prostate cancer biochemical recurrence. J Urol. 2011;185(3):848–54.
Schröder FH, Tombal B, Miller K, Boccon‐Gibod L, Shore ND, Crawford ED, et al. Changes in alkaline phosphatase levels in patients with prostate cancer receiving degarelix or leuprolide: results from a 12‐month, comparative, phase III study. BJU Int. 2010;106(2):182–7.
Hong JH, Kim IY. Nonmetastatic castration-resistant prostate cancer. Korean J Urol. 2014;55(3):153–60.
Nelson JB, Love W, Chin JL, Saad F, Schulman CC, Sleep DJ, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone‐refractory prostate cancer. Cancer 2008;113(9):2478–87.
Miller K, Moul J, Gleave M, Fizazi K, Nelson J, Morris T, et al. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2013;16(2):187.
Madan RA, Gulley JL, Schlom J, Steinberg SM, Liewehr DJ, Dahut WL, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14(14):4526–31.
Beinart G, Rini BI, Weinberg V, Small EJ. Antigen-presenting cells 8015 (Provenge®) in patients with androgen-dependent, biochemically relapsed prostate cancer. Clin Prostate Cancer. 2005;4(1):55–60.
Ogita S, Tejwani S, Heilbrun L, Fontana J, Heath E, Freeman S, et al. Pilot phase II trial of bevacizumab monotherapy in nonmetastatic castrate-resistant prostate cancer. ISRN oncology. 2012;2012:242850
Ito K, Kimura T, Onuma H, Tabata R, Shimomura T, Miki K, et al. Does docetaxel prolong survival of patients with non-metastatic castration-resistant prostate cancer? Prostate 2018 May;78(7):498-505.
Ryan CJ, Smith MR, De Bono JS, Molina A, Logothetis CJ, De Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. New Engl J Med. 2013;368(2):138–48.
De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. New Engl J Med. 2011;364(21):1995–2005.
Ryan CJ, Crawford ED, Shore ND, Underwood W, Wang J, DePalantino J, et al. Effect of abiraterone acetate and low-dose prednisone on PSA in patients with nonmetastatic castration-resistant prostate cancer: The results from IMAAGEN core study. American Society of Clinical Oncology; 2014 32:15_suppl, 5086–86.
Ryan CJ, Crawford ED, Shore ND, Underwood W, Londhe A, Black SC, et al. IMAAGEN trial update: Effect of abiraterone acetate and low dose prednisone on PSA and radiographic disease progression in patients with non-metastatic castration-resistant prostate cancer. American Society of Clinical Oncology; 2015;33:15_suppl, 5053–53.
Hussain M, Corn P, Michaelson D, Hammers H, Alumkal J, Ryan C, et al. 124 Activity and safety of the investigational agent orteronel (ortl, TAK-700) in men with nonmetastatic castration-resistant prostate cancer (CRPC) and rising prostate-specific antigen (PSA): Results of a phase 2 study. Eur Urol Suppl. 2012;11(1):e124–ea.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. New Engl J Med. 2014;371(5):424–33.
Scher HI, Fizazi K, Saad F, Taplin M-E, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New Engl J Med. 2012;367(13):1187–97.
Penson DF, Armstrong AJ, Concepcion R, Agarwal N, Olsson C, Karsh L, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34(18):2098–106.
Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465–74.
Jung ME, Ouk S, Yoo D, Sawyers CL, Chen C, Tran C, et al. Structure− activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC). J Med Chem. 2010;53(7):2779–96.
Smith MR, Antonarakis ES, Ryan CJ, Berry WR, Shore ND, Liu G, et al. Phase 2 study of the safety and antitumor activity of apalutamide (ARN-509), a potent androgen receptor antagonist, in the high-risk nonmetastatic castration-resistant prostate cancer cohort. Eur Urol. 2016;70(6):963–70.
Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018 Apr 12;378(15):1408–18.
Schweizer M, Zhou X, Wang H, Yang T, Shaukat F, Partin A, et al. Metastasis-free survival is associated with overall survival in men with PSA-recurrent prostate cancer treated with deferred androgen deprivation therapy. Ann Oncol. 2013;24(11):2881–6.
Xie W, Regan M, Buyse M, Halabi S, Kantoff P, Sartor O, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017;35(27):3097–114.
Shore ND. Darolutamide (ODM-201) for the treatment of prostate cancer. Expert Opin Pharmacother. 2017;18(9):945–52.
Funding
Chritopher P. Evans has received research funding, honorarium, consulting and speaking relationships with Medivation, Janssen, Astellas and Sanofi in the past.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Sigfred Ian Alpajaro and Jared A.K. Harris do not have any conflict of interest.
Rights and permissions
About this article
Cite this article
Alpajaro, S.I.R., Harris, J.A.K. & Evans, C.P. Non-metastatic castration resistant prostate cancer: a review of current and emerging medical therapies. Prostate Cancer Prostatic Dis 22, 16–23 (2019). https://doi.org/10.1038/s41391-018-0078-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-018-0078-1
This article is cited by
-
Exploring the mechanism of action of Sparganii Rhizoma-Curcumae Rhizoma for in treating castration-resistant prostate cancer: a network-based pharmacology and experimental validation study
Scientific Reports (2024)
-
The Hospitalization-Related Costs of Adverse Events for Novel Androgen Receptor Inhibitors in Non-Metastatic Castration-Resistant Prostate Cancer: An Indirect Comparison
Advances in Therapy (2022)
-
Synthesis, preclinical evaluation, and first-in-human study of Al18F-PSMA-Q for prostate cancer imaging
European Journal of Nuclear Medicine and Molecular Imaging (2022)
-
Abiraterone and enzalutamide had different adverse effects on the cardiovascular system: a systematic review with pairwise and network meta-analyses
Prostate Cancer and Prostatic Diseases (2021)
-
Physician preferences for non-metastatic castration-resistant prostate cancer treatment
BMC Urology (2020)