Abstract
Background
Management of active surveillance (AS) in low-risk prostate cancer (PCa) patients could be improved with new biomarkers, such as the 4Kscore test. We analyze its ability to predict tumor reclassification by upgrading at the confirmatory biopsy at 6 months.
Methods
Observational, prospective, blinded, and non-randomized study, within the Spanish National Registry on AS (AEU/PIEM/2014/0001; NCT02865330) with 181 patients included after initial Bx and inclusion criteria: PSA ≤10 ng/mL, cT1c-T2a, Grade group 1, ≤2 cores, and ≤5 mm/50% length core involved. Central pathological review of initial and confirmatory Bx was performed on all biopsy specimens. Plasma was collected 6 months after initial Bx and just before confirmatory Bx to determine 4Kscore result. In order to predict reclassification defined as Grade group ≥2, we analyzed 4Kscore, percent free to total (%f/t) PSA ratio, prostate volume, PSA density, family history, body mass index, initial Bx, total cores, initial Bx positive cores, initial Bx % of positive cores, initial Bx maximum cancer core length and initial Bx cancer % involvement. Wilcoxon rank-sum test, non-parametric trend test or Fisher’s exact test, as appropriate established differences between groups of reclassification.
Results
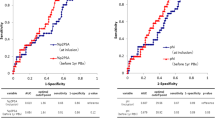
A total of 137 patients met inclusion criteria. Eighteen patients (13.1%) were reclassified at confirmatory Bx. The %f/t PSA ratio and 4Kscore showed differences between the groups of reclassification (Yes/No). Using 7.5% as cutoff for the 4Kscore, we found a sensitivity of 89% and a specificity of 29%, with no reclassifications to Grade group 3 for patients with 4Kscore below 7.5% and 2 (6%) missed Grade group 2 reclassified patients. Using this threshold value there is a biopsy reduction of 27%. Additionally, 4Kscore was also associated with changes in tumor volume.
Conclusions
Our preliminary findings suggest that the 4Kscore may be a useful tool in the decision-making process to perform a confirmatory Bx in active surveillance management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wilt TJ, Jones KM, Barry MJ, Andriole GL, Culkin D, Wheeler T, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132–42.
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24.
Rubio-Briones J, Borque-Fernando A, Esteban-Escaño LM, Martínez-Breijo S, Medina-López R, Hernández V, et al. Variability in the multicentre National Registry in Active Surveillance; a questionnaire for urologists. Actas Urológicas Españolas. 2018. https://doi.org/10.1016/j.acuro.2018.01.007.
Bruinsma SM, Zhang L, Roobol MJ, Bangma CH, Steyerberg EW, Nieboer D, et al. The Movember Foundation’s GAP3 cohort: a profile of the largest global prostate cancer active surveillance database to date. BJU Int. 2018;121:737–44.
Drost F-JH, Rannikko A, Valdagni R, Pickles T, Kakehi Y, Remmers S, et al. Can active surveillance really reduce the harms of overdiagnosing prostate cancer? A reflection of real life clinical practice in the PRIAS study. Transl Androl Urol. 2018;7:98–105.
Schoots IG, Petrides N, Giganti F, Bokhorst LP, Rannikko A, Klotz L, et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol. 2015;67:627–36.
Barrett T, Haider MA. The emerging role of MRI in prostate cancer active surveillance and ongoing challenges. Am J Roentgenol. 2017;208:131–9.
Siddiqui MM, Rais-Bahrami S, Truong H, Stamatakis L, Vourganti S, Nix J, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713–9.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH. et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–77.
Parekh DJ, Punnen S, Sjoberg DD, Asroff SW, Bailen JL, Cochran JS, et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol. 2015;68:464–70.
Van Neste L, Hendriks RJ, Dijkstra S, Trooskens G, Cornel EB, Jannink SA, et al. Detection of high-grade prostate cancer using a urinary molecular biomarker-based risk score. Eur Urol. 2016;70:740–8.
Hendriks RJ, van der Leest MMG, Dijkstra S, Barentsz JO, Van Criekinge W, Hulsbergen-van de KaaCA, et al. A urinary biomarker-based risk score correlates with multiparametric MRI for prostate cancer detection. Prostate. 2017;77:1401–7.
Mottet N, Bellmunt J, Briers E, Bolla M, Bourke L, Cornford P, et al. EAU - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer 2018. 2018 http://uroweb.org/guideline/prostate-cancer/.
Mohler JL, Lee RJ, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, et al. Prostate cancerv2.2018 (NCCN Guidelines). 2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A contemporary prostate cancer grading system: a validated alternative to the gleason score. Eur Urol. 2016;69:428–35.
Rubio-Briones J, Borque A, Esteban LM, Iborra I, López PA, Gil JM. et al. Preliminary results of the Spanish Association of Urology National Registry in Active Surveillance for prostate cancer. Actas Urol Esp. 2016;40:3–10.
Punnen S, Pavan N, Parekh DJ. Finding the wolf in sheep’s clothing: the 4Kscore is a novel blood test that can accurately identify the risk of aggressive prostate cancer. Rev Urol. 2015;17:3–13.
Iremashvili V, Burdick-Will J, Soloway MS. Improving risk stratification in patients with prostate cancer managed by active surveillance: a nomogram predicting the risk of biopsy progression. BJU Int. 2013;112:39–44.
Voigt JD, Dong Y, Linder V, Zappala S. Use of the 4Kscore test to predict the risk of aggressive prostate cancer prior to prostate biopsy: overall cost savings and improved quality of care to the US healthcare system. Rev Urol. 2017; 19.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5434830/pdf/RIU019001_0001.pdf. Accessed 9 Jan 2018.
Stattin P, Vickers AJ, Sjoberg DD, Johansson R, Granfors T, Johansson M, et al. Improving the specificity of screening for lethal prostate cancer using prostate-specific antigen and a panel of kallikrein markers: a nested case-control study. Eur Urol. 2015;68:207–13.
Punnen S, Zappala S, Palou J, Sjoberg D, Mathur V, Roberts R, et al. Among men with low-grade prostate cancer on prostate biopsy, the 4Kscore predicts the presence of more aggressive prostate cancer. In: 30th Annual EAU Congress Madrid. Eur Urol, Suppl. 2015;14:e433.
Punnen S, Zappala S, Mathur V, Reeve M, Parekh D. MP77-20 among men with low-grade prostate cancer on prostate biopsy, the 4Kscore predicts more aggressive prostate cancer at prostatectomy. J Urol Suppl. 2015;193:e1001
Lin DW, Newcomb LF, Brown MD, Sjoberg DD, Dong Y, Brooks JD, et al. Evaluating the four Kallikrein panel of the 4Kscore for prediction of high-grade prostate cancer in men in the Canary prostate active surveillance study. Eur Urol. 2017;72:448–54.
Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Borque-Fernando, Á., Rubio-Briones, J., Esteban, L.M. et al. Role of the 4Kscore test as a predictor of reclassification in prostate cancer active surveillance. Prostate Cancer Prostatic Dis 22, 84–90 (2019). https://doi.org/10.1038/s41391-018-0074-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-018-0074-5
This article is cited by
-
Independent validation of a pre-specified four-kallikrein marker model for prediction of adverse pathology and biochemical recurrence
British Journal of Cancer (2022)
-
A pre-specified model based on four kallikrein markers in blood improves predictions of adverse pathology and biochemical recurrence after radical prostatectomy
British Journal of Cancer (2020)
-
Role of multiparametric magnetic resonance imaging for patients under active surveillance for prostate cancer: a systematic review with diagnostic meta-analysis
Prostate Cancer and Prostatic Diseases (2019)
-
Active Surveillance beim Prostatakarzinom
Der Urologe (2019)