Abstract
Background
The significance of arousal in apnea termination in preterm infants is not known.
Methods
We investigated the appearance of arousals from sleep with polysomnography for 21 preterm infants at a median age of 36 gestational weeks.
Results
The polysomnographic appearance of sleep was fragmented by frequent arousals. The number of spontaneous arousals unrelated to apneas was 18 per hour in sleep; higher in rapid eye movement (REM) sleep than in non-REM sleep (p < 0.001). Eighty-two percent of arousals were regarded as spontaneous, and 18% were related to apneas. In turn, arousal followed 5% of all apneas; 30% of mixed, 2% of central, and 20% of long apneas defined as apnea of prematurity. Apneas without an arousal led to lower oxygen saturation levels than those followed by an arousal (p < 0.001). Mixed apneas with an arousal had stronger breathing effort and a higher number of breaths compared with apneas without an arousal (p < 0.05).
Conclusions
In preterm infants, frequent spontaneous arousals or arousal-type phenomena make the polysomnographic appearance of sleep fragmented. However, even long apneas or hypoxia commonly fail to elicit arousals or any sign of sleep interruption. Our findings suggest that arousal appears not to be the main mechanism for apnea termination in preterm infants.
Impact
-
Polysomnographic appearance of sleep in preterm infants is fragmented by arousals.
-
Contrary to older children and adults, arousal to apnea is uncommon in preterm infants.
-
Even long mixed apneas with desaturation mostly fail to elicit an arousal response.
-
In preterm infants, apnea termination appears not to depend on an arousal.
-
Low arousability is suggested to be caused by a low ventilation response to hypoxia.
Similar content being viewed by others
Introduction
Apneas are common during sleep in infants. Most apneas are isolated short apneas. In addition, preterm infants commonly express long apneas with hypoxia or bradycardia called apnea of prematurity (AOP), and short apneas separated by hyperpneic episodes, called periodic breathing (PB).1,2,3,4 In adults, obstructive and mixed apneas are often terminated by arousal from sleep. In the past, arousal was considered obligatory to end an obstructive apnea, but recent studies have shown that most patients do not have or need cortical arousal to terminate these apneas.5,6,7 In infants and children, the arousal response to obstructive and mixed apneas is less frequent than in adults, but it increases with age.8,9 Like in adults, central apneas and PB rarely cause an arousal or sleep disturbance.5,10 We do not know much about arousal responses to apneas in preterm infants.
Spontaneous arousals refer to arousals from sleep with no detected external or internal cause.11 Most studies regarding arousal responses of preterm infants have focused on these spontaneous arousals, on the effects of position on arousals, or arousal threshold by external stimulus.12,13,14,15,16,17,18 There are only two small studies in preterm infants specifically addressing arousal responses to apneas.19,20
The aim of our study was to investigate the characteristics of apnea arousals in preterm infants with polysomnography (PSG). We were especially interested in the characteristics of long apneas, and whether their presence could be explained by a failure of arousal response. However, soon after the beginning of the analysis, we discovered the high frequency of spontaneous arousals in these preterm infants. Thus, the analysis of these spontaneous arousals was included in the study.
Methods
Study design and patients
We recorded PSG in 21 preterm infants cared for in the neonatal units of Helsinki and Uusimaa Hospital District, Finland. At the time of the study, the included infants were clinically stable with no respiratory support, supplemental oxygen, or caffeine treatment. The PSG recordings were performed at a median 36 (interquartile range (IQR) 35–36) weeks of gestational age, at 4.7 (IQR 2.8–7.1) weeks of age. Detailed demographic data are presented in Table 1. The recordings of these infants were also used to investigate the effects of caffeine and supplemental oxygen on PB and apneas in preterm infants.4 According to cardiorespiratory monitoring (IntelliVue MX 700 and IntelliVue Information Center data collection, Philips, The Netherlands) all infants presented PB or long apneas. Furthermore, the treating physician assessed these infants to need commencement of caffeine therapy for apneas or excessive PB. The PSG recordings used in the current study were performed prior to (re)commencing caffeine treatment or supplemental oxygen. Of the infants studied, 14 (67%) had received previous caffeine treatment. However, this treatment had been discontinued at a median 8 (IQR 7–11) days prior to the study.
The local ethics committee of the University of Helsinki approved the study protocol and parents provided written consent prior to participation.
Polysomnography
The PSG protocol followed the current American Academy of Sleep Medicine (AASM) recommendations and comprised monitoring of four electroencephalography (EEG) channels (C4-M1/A1, Cz-Fz, Cz-O2, O2-M1/A1), right and left electro-oculography (EOG) channels, chin and diaphragm electromyography (EMG), nasal airflow using a pressure sensor, respiratory movements using an abdominal band, electrocardiography (ECG), pulse oximeter oxygen saturation (SpO2) with a 4-s averaging interval, and end-tidal carbon dioxide.21 For the recordings we used Siesta PSG equipment (Compumedics, Abbotsford, Australia).
Sleep staging
The PSG recordings were analyzed and scored by an experienced scorer (T.K.) using Embla RemLogic PSG software (Natus Medical Inc., California, USA) with additional special-purpose software. The sleep stage analysis was performed visually, recognizing non-rapid eye movement sleep (NREM) sleep, rapid eye movement sleep (REM) sleep, and wakefulness. For sleep staging, we used the current AASM infant sleep stage criteria and the recommendations of Grigg-Dammberger and associates,21,22 but we did not use the transitional stage (T) classification, and scored corresponding epochs either as NREM or REM based on chin EMG measurements.
Apnea definition
We determined central respiratory pauses longer than two breathing cycles and lasting 4 s or more as apneas. All obstructive breaths were noted regardless of the duration of apnea. Central, obstructive, and mixed apneas were recognized based on airflow, diaphragm EMG activity, and respiratory movements. Apneas with no breathing movements were defined as central apneas, apneas with breathing movements but no airflow were determined as obstructive apneas, and respiratory pauses that commenced as central but showed obstructive respiratory movements with no airflow as mixed apneas. An apnea was also defined as AOP if it caused a heart rate decrease to <100 beats/min, or a drop in SpO2 to under 80%, or it lasted longer than 20 s.1 Breathing effort (inspiratory effort) was visually scored from the PSG analysis according to the diaphragm EMG in obstructive and mixed apneas by a scale of 0 (no effort)–1–2 (maximal effort).
Arousal definition
There is no consensus for scoring arousals in the preterm period. Therefore, we applied the criteria of The International Pediatric Workgroup on Arousals for infants aged 1–6 months.11 However, due to immaturity of the EEG and EEG movement artifacts, it was not particularly helpful for detecting arousals or awakenings. Thus, we did not differentiate between cortical and subcortical arousals nor did we use computational signal analysis methods. Heart rate changes were mainly related to apnea and hypoxia, and they did not seem to correlate with arousals.
For the study purposes, an arousal from sleep (REM or NREM) was defined as a period of 3 s or more of
-
sustained increase in chin and diaphragm EMG from baseline recording excluding sucking of the dummy and/ or
-
gross body movements causing artifacts in ECG, EEG, and respiratory signals, especially if increased activity in chin and diaphragm EMG preceded or lasted longer than gross body movements.
An arousal was defined as a long arousal or awakening if the increase in chin and diaphragm EMG or clear gross body movements lasted >15 s. To be determined as an apnea arousal, the arousal needed to appear during an apnea, or within 5 s after the end of an apnea.
Statistical methods
The statistical analysis was done with SPSS® Statistics software version 24 (IBM, New York, USA). For pairwise comparisons we used the nonparametric paired-samples sign test. For comparisons of multiple related groups (hypoxia analysis), we used the nonparametric Friedman test. We chose nonparametric tests due to the number of study patients and the non-normal distribution of the variables. The level of significance was set at p < 0.05.
Results
The 21 studied infants were born at a median 31 (IQR 28–34) weeks of gestation with a median birth weight of 1610 (IQR 1140–2190) g. They were studied at a median 36 (IQR 35–36) weeks of gestational age. The demographic data and sleep stage distribution are presented in Table 1.
Spontaneous arousal-type phenomena in PSG recordings were frequent, and this made the PSG appearance of sleep fragmented (Table 2). The total arousal frequency was 21 per hour of sleep. However, only 18% of these arousals were preceded by apneic events, that is, were apnea arousals. The PSG appearance of both spontaneous and apnea arousals was significantly more frequent in REM sleep than in NREM sleep (p < 0.05 and p < 0.001) (Table 2). The distribution of short arousals and long arousals or awakenings is presented in Table 2.
We scored a total amount of 3993 apneas in the PSG recordings. Of all the apneas, 88% (3504) were central, 1% (52) was obstructive, and 11% (437) were mixed events. Of these apneas, only 5% (204) ended in an arousal, 3% (127) during REM, and 2% (77) during NREM sleep. Although infrequent, apnea arousals were more common in REM than in NREM sleep (p < 0.001). This difference was not present when separating apnea types. Arousal to apnea was scarce, and frequencies in different types of apneas are presented in Table 3.
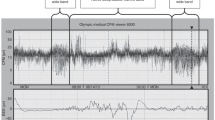
Differences in characteristics of apneas with or without arousal are presented in Tables 4 and 5. The level of hypoxia (oxygen saturation levels) did not correlate with breathing effort in apneas with or without arousals (Fig. 1). Figures 2 and 3 show typical appearances of a mixed apnea with and without an arousal in REM sleep.
This scatter plot figure shows that despite breathing effort and oxygen saturation levels being lower in mixed apneas without arousal compared to those with arousal (Tables 4 and 5), breathing effort did not correlate to oxygen saturation levels in mixed apneas with or without arousals. SpO2 pulse oximeter oxygen saturation.
Note the bradycardia and desaturation caused by the apnea and changes in the diaphragm and chin EMG and the EEG channels during the arousal. EEG electroencephalogram, EOG electro-oculogram, EMG chin and diaphragm skin surface electromyograms, Resp respiratory movements, SpO2 pulse oximeter oxygen saturation, EtCO2 carbon dioxide content of exhaled air, ECG electrocardiogram.
Arousal to hypoxia was infrequent (Table 6). Only 5% of all SpO2 drops to <95% led to arousal from sleep. Furthermore, only 5% of desaturations of at least 5% units from baseline SpO2 levels led to arousal from sleep. The degree of the drop in baseline SpO2 level or desaturation did not significantly affect the arousal rate to hypoxia. The frequency of arousal to hypoxia was higher in REM sleep than in NREM sleep (Fig. 4).
Discussion
This study has three main findings. The first finding is that spontaneous arousals or arousal-type phenomena are frequent in the late-preterm period. Due to movements or movement arousals, the PSG appearance of sleep is fragmented. The second finding is that despite of frequent spontaneous movements, movement arousals or other indicators of sleep interruption, even long central and mixed apneas of >15 s were infrequently followed by an arousal or any arousal-type phenomena. The third finding is that hypoxia is not a potent stimulus for arousal in preterm infants.
Central and mixed apneas that failed to cause an arousal led to lower oxygen saturation levels than those followed by an arousal. On the other hand, mixed apneas followed by an arousal had stronger breathing efforts compared with mixed apneas not accompanied by arousals. These results support the idea that the tendency to long apneas in preterm infants is related to low responsiveness to hypoxia together with easily collapsible upper airways. Apnea-related hypoxia does not seem to elicit a high stimulus to arousal.
Arousal to apnea in preterm infants
Concurring with previous data, we showed that apneas often fail to elicit an arousal in preterm infants.19,20 In our study, apnea arousals were more frequent in REM sleep than in NREM sleep. This is most likely explained by different types of apneas. Most central apneas occurred in NREM sleep, whereas most mixed apneas and AOPs in REM sleep.4
There are two previous studies addressing the appearance of apnea arousals during the preterm period.19,20 Thoppil and associates studied arousal responses based on video recordings and cardiorespiratory polygraphy ten non-apneic and ten apneic preterm infants at the age of 32–34 weeks of gestation. They discovered very similar findings and numbers to our data. In their study, in apneic preterm infants arousal followed only 3% (3/100) of central and 27% (13/49) of mixed apneas, 7% (10/138) of short apneas <15 s, and 50% (6/12) of long apneas over 15 s. In our data, 47% (21/45) of long mixed apneas over 15 s, and 39% (12/31) of AOPs over 15 s were followed by and arousal.
The second study addressing arousal or arousal-type phenomena to apneas in preterm infants is by Curzi-Dascalova and associates.20 They investigated PSG data without chin EMG recordings in five preterm infants at 33–34 weeks of gestation. They found that most apneas, especially central ones, did not cause physiological changes indicating an arousal. In their study, 9% of central apneas and 18% of obstructive and mixed apneas were followed by movements.20
Our study suggests that an arousal response is not the main mechanism of apnea termination in preterm infants, especially regarding central apneas. Our results coincide with previous data of preterm and older infants.10,19,20 However, obstructed breaths were more commonly followed by an arousal in the same way as previously observed in preterm and older infants, children, and adults.5,10,19,20 The increased probability of obstructive breaths to lead to arousal may be due to the stimulating effect of upper airways or respiratory muscles.23
Arousal to hypoxia
PB and apneas commonly cause desaturations and intermittent hypoxia in preterm and term infants.2,4,24 However, this apnea-related hypoxia seems to rarely lead to arousal. In our study, the degree of hypoxia or desaturation did not affect arousability from sleep, and arousal to hypoxia was scarce. In contrast to animal studies, in infants hypoxia appears to be a more potent cause of an arousal in REM sleep than in NREM sleep.25,26 This may be due to a more rapid decrease in oxygen saturation during apnea in REM than in NREM sleep.27,28 In our study, a drop in baseline SpO2 to <95% or a desaturation of >5% units from baseline SpO2 level was more potent in causing an arousal in REM sleep than in NREM sleep, concurring with previous data. We speculate that the reason for low arousability to hypoxia, and furthermore to apneas, may be the low, downwards shifted biphasic hypoxic ventilatory response in preterm and term infants.26,29
Spontaneous arousals
The high frequency of arousals or arousal-type phenomena of 21 per hour of sleep in this study is similar to observed in term infants.30 Like in previous studies,8,12,13,30,31 we observed arousals to be more frequent in REM than in NREM sleep. However, spontaneous arousals and lack of long periods of uninterrupted sleep were also common in NREM sleep.
Definition of sleep and arousal
There is no consensus of the criteria for sleep staging or arousal definition in the preterm period. We find the literature to support the use of NREM and REM for sleep staging instead of the behavioral sleep states of quiet, active, and intermediate sleep.21,22
The International Pediatric Workgroup on Arousals published their recommendations for the scoring of arousals for healthy term infants aged 1 to 6 months in 2005.11 These rules were established primarily to enable the comparisons of varying studies investigating arousals, and no consensus addressing apnea arousals specifically was made. The applicability of these rules has also been assessed in preterm infants before the release of the recommendation, but was not included in the statement.32 Therefore, we used modified criteria for arousal definition.
Our primary target was to investigate the response of arousal to apnea. However, the PSG appearance of spontaneous arousal-type phenomena was high, requiring us to include the analysis of these arousals. Before 2 to 3 months of age, REM sleep is characterized by spontaneous movements, that is, muscle twitches and gross body and extremity movements.33 In preterm infants, we find it hard with the current PSG methodology to separate autonomic or cortical arousals from spontaneous movement not causing disturbances of sleep. Preterm infants express frequent spontaneous motor activity in REM sleep. However, frequent arousal appearance in PSG recordings was noted also in NREM sleep.22
We recognized arousal characteristics in these preterm infants in apnea-related arousals and applied these criteria also to spontaneous arousals. All PSG signals were used for arousal detection. Because of immaturity of the EEG it was not helpful as a criterion for arousal or awakening. EEG changes mainly reflected body movement artifacts and showed no obvious phase shift changes. Heart rate changes were mainly related to apneas and hypoxia and did not appear to correlate with arousals. Chin and diaphragm EMG changes appeared most reliable for the detection of arousals.
Limitations of the study
Our study has limitations. As discussed above, there is no consensus for the definition of arousal for preterm infants. Although we may use the recommendations of The International Pediatric Workgroup for healthy term infants from age 1 to 6 months, the rules are not fully applicable for preterm infants.11 This makes comparison of different studies difficult and may influence the results and interpretations. More precise recommendations for assessing and scoring arousals in preterm infants is needed.
As in the preceding studies addressing apnea arousals in preterm infants, the number of infants in our study is still rather small. Of the 21 studied infants, five infants had mild BPD and three infants had suffered from germinal matrix or intraventricular hemorrhage. However, this population represents a typical preterm infant population. During the time of the study, all infants were stable without respiratory support or significant neurological symptoms or findings. Therefore, we believe these baseline conditions have not had a significant effect on our study results.
Conclusion
Among preterm infants, the polysomnographic appearance of sleep is fragmented by frequent arousal-type phenomena. Arousals appear dominantly in REM sleep. Apneas or hypoxia rarely terminate in an arousal, and even long central and mixed apneas with hypoxia frequently fail to elicit an arousal or any indication of sleep interruption. Thus, in many cases, the mechanism of apnea termination in preterm infants appears not to be dependent on arousal.
References
Eichenwald, E. C. Apnea of prematurity. Pediatrics 137, 1–7 (2016).
Poets, C. F. & Southall, D. P. Patterns of oxygenation during periodic breathing in preterm infants. Early Hum. Dev. 26, 1–12 (1991).
Oliveira, A. J., Nunes, M. L., Fojo-Olmos, A., Reis, F. M. & da Costa, J. C. Clinical correlates of periodic breathing in neonatal polysomnography. Clin. Neurophysiol. 115, 2247–2251 (2004).
Seppa-Moilanen, M., Andersson, S., Rantakari, K., Mikkola, K. & Kirjavainen, T. Caffeine and supplemental oxygen effectively suppress periodic breathing with only minor effects during long episodes of apnoea in preterm infants. Acta Paediatr. 108, 443–451 (2018).
Berry, R. B. & Gleeson, K. Respiratory arousal from sleep: mechanisms and significance. Sleep 20, 654–675 (1997).
Eckert, D. J. & Younes, M. K. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J. Appl. Physiol. 116, 302–313 (2014).
Younes, M. Pathogenesis of obstructive sleep apnea. Clin. Chest Med. 40, 317–330 (2019).
Franco, P. et al. Arousal from sleep mechanisms in infants. Sleep. Med. 11, 603–614 (2010).
Katz, E. S., Mitchell, R. B. & D’Ambrosio, C. M. Obstructive sleep apnea in infants. Am. J. Respir. Crit. Care Med. 185, 805–816 (2012).
McNamara, F., Issa, F. G. & Sullivan, C. E. Arousal pattern following central and obstructive breathing abnormalities in infants and children. J. Appl. Physiol. 81, 2651–2657 (1996).
International Paediatric Work Group on Arousals. The scoring of arousals in healthy term infants (between the ages of 1 and 6 months). J. Sleep Res. 14, 37–41 (2005).
Horne, R. S., Sly, D. J., Cranage, S. M., Chau, B. & Adamson, T. M. Effects of prematurity on arousal from sleep in the newborn infant. Pediatr. Res. 47, 468–474 (2000).
Horne, R. S. et al. Apnoea of prematurity and arousal from sleep. Early Hum. Dev. 61, 119–133 (2001).
Bhat, R. Y. et al. Effect of prone and supine position on sleep, apneas, and arousal in preterm infants. Pediatrics 118, 101–107 (2006).
Goto, K., Maeda, T., Mirmiran, M. & Ariagno, R. Effects of prone and supine position on sleep characteristics in preterm infants. Psychiat. Clin. Neurosci 53, 315–317 (1999).
Horne, R. S., Bandopadhayay, P., Vitkovic, J., Cranage, S. M. & Adamson, T. M. Effects of age and sleeping position on arousal from sleep in preterm infants. Sleep 25, 746–750 (2002).
Richardson, H. L. & Horne, R. S. Arousal from sleep pathways are affected by the prone sleeping position and preterm birth: preterm birth, prone sleeping and arousal from sleep. Early Hum. Dev. 89, 705–711 (2013).
Modesto, I. F. et al. Effect of sleeping position on arousals from sleep in preterm infants. J. Spec. Pediatr. Nurs. 21, 131–138 (2016).
Thoppil, C. K., Belan, M. A., Cowen, C. P. & Mathew, O. P. Behavioral arousal in newborn infants and its association with termination of apnea. J. Appl Physiol. 70, 2479–2484 (1991).
Curzi-Dascalova, L., Bloch, J., Vecchierini, M., Bedu, A. & Vignolo, P. Physiological parameters evaluation following apnea in healthy premature infants. Biol. Neonate. 77, 203–211 (2000).
Berry, R. B., Quan, S. F., Abreu, A. R., Bibbs, M. L., DelRosso, L. The AASM Manual for the Scoring of Sleep and Associated events: Rules, Terminology and Technical Specifications, Version 2.6. ad. (American Academy of Sleep Medicine, Darien, 2020).
Grigg-Damberger, M. M. The visual scoring of sleep in infants 0 to 2 months of age. J. Clin. Sleep. Med. 12, 429–445 (2016).
Whitehead, K., Jones, L., Laudiano-Dray, M. P., Meek, J. & Fabrizi, L. Event-related potentials following contraction of respiratory muscles in pre-term and full-term infants. Clin. Neurophysiol. 130, 2216–2221 (2019).
Urlesberger, B. et al. Changes in cerebral blood volume and cerebral oxygenation during periodic breathing in term infants. Neuropediatrics 31, 75–81 (2000).
Parslow, P. M., Harding, R., Adamson, T. M. & Horne, R. S. Of sleep state and postnatal age on arousal responses induced by mild hypoxia in infants. Sleep 27, 105–109 (2004).
Verbeek, M. M. et al. Arousal and ventilatory responses to mild hypoxia in sleeping preterm infants. J. Sleep. Res. 17, 344–353 (2008).
Horne, R. S., Parslow, P. M. & Harding, R. Postnatal development of ventilatory and arousal responses to hypoxia in human infants. Respir. Physiol. Neurobiol. 149, 257–271 (2005).
Darnall, R. A. The carotid body and arousal in the fetus and neonate. Respir. Physiol. Neurobiol. 185, 132–143 (2013).
Rigatto, H. Control of ventilation in the newborn. Annu. Rev. Physiol. 46, 661–674 (1984).
Montemitro, E. et al. Maturation of spontaneous arousals in healthy infants. Sleep 31, 47–54 (2008).
McNamara, F., Lijowska, A. S. & Thach, B. T. Spontaneous arousal activity in infants during NREM and REM sleep. J. Physiol. 538, 263–269 (2002).
Zotter, H., Urlesberger, B., Muller, W. & Kerbl, R. How to score arousals in preterm infants? Can we use recommendations of the Pediatric Wake-up Club? Wien. Klin. Wochenschr. 115, 867–870 (2003).
Graven, S. Sleep and brain development. Clin. Perinatol. 33, 693–706 (2006). vii.
Acknowledgements
We thank the study patients and their families for participation in our study. We also thank the staff at Jorvi Hospital and Kätilöopisto Maternity Hospital neonatal wards of the Helsinki and Uusimaa Hospital district for their contribution and help in recruiting the study patients and aiding in the PSG studies. We thank the Helsinki University biostatistics consulting office for their consultation in the statistical analysis. We thank the Finnish Foundation for Pediatric Research for the financial support to this study. M.S.-M. received a personal grant from the Finnish Foundation for Pediatric Research.
Author information
Authors and Affiliations
Contributions
M.S.-M. contributed to the study plan, performed and analyzed the sleep studies and statistics, drafted the initial manuscript, and approved the final manuscript as submitted. S.A. contributed to the study plan, reviewed and revised the manuscript, and approved the final manuscript as submitted. T.K. conceptualized and designed the study plan, performed and analyzed the sleep studies and statistics, drafted the initial manuscript, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Parent consent
Parents of the study infants provided written consent prior to participation in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seppä-Moilanen, M., Andersson, S. & Kirjavainen, T. Spontaneous and apnea arousals from sleep in preterm infants. Pediatr Res 89, 1261–1267 (2021). https://doi.org/10.1038/s41390-020-1068-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-1068-2
This article is cited by
-
Caffeine is a respiratory stimulant without effect on sleep in the short-term in late-preterm infants
Pediatric Research (2022)