Abstract
Objective
To test the hypothesis that a feeding bundle concurrent with acid suppression is superior to acid suppression alone in improving gastroesophageal reflux disease (GERD) attributed-symptom scores and feeding outcomes in neonatal ICU infants.
Methods
Infants (N = 76) between 34 and 60 weeks’ postmenstrual age with acid reflux index > 3% were randomly allocated to study (acid-suppressive therapy + feeding bundle) or conventional (acid-suppressive therapy only) arms for 4 weeks. Feeding bundle included: total fluid volume < 140 mL/kg/day, fed over 30 min in right lateral position, and supine postprandial position. Primary outcome was independent oral feeding and/or ≥6-point decrease in symptom score (I-GERQ-R). Secondary outcomes included growth (weight, length, head circumference), length of hospital stay (LOHS, days), airway (oxygen at discharge), and developmental (Bayley scores) milestones.
Results
Of 688 screened: 76 infants were randomized and used for the primary outcome as intent-to-treat, and secondary outcomes analyzed for 72 infants (N = 35 conventional, N = 37 study). For study vs. conventional groups, respectively: (a) 33% (95% CI, 19−49%) vs. 44% (95% CI, 28−62%), P = 0.28 achieved primary outcome success, and (b) secondary outcomes did not significantly differ (P > 0.05).
Conclusions
Feeding strategy modifications concurrent with acid suppression are not superior to PPI alone in improving GERD symptoms or discharge feeding, short-term and long-term outcomes.
Impact
-
Conservative feeding therapies are thought to modify GERD symptoms and its consequences. However, in this randomized controlled trial in convalescing neonatal ICU infants with GERD symptoms, when controlling for preterm or full-term birth and severity of esophageal acid reflux index, the effectiveness of acid suppression plus a feeding modification bundle (volume restriction, intra- and postprandial body positions, and prolonged feeding periods) vs. acid suppression alone, administered over a 4-week period was not superior in improving symptom scores or feeding outcomes.
-
Restrictive feeding strategies are of no impact in modifying GERD symptoms or clinically meaningful outcomes. Further studies are needed to define true GERD and to identify effective therapies in modifying pathophysiology and outcomes.
-
The improvement in symptoms and feeding outcomes over time irrespective of feeding modifications may suggest a maturational effect. This study justifies the use of placebo-controlled randomized clinical trial among NICU infants with objectively defined GERD.
Similar content being viewed by others
Introduction
Differentiating gastroesophageal reflux (physiological, GER) from GER disease (pathological, GERD) remains a challenge in ICU infants.1,2,3,4,5 Troublesome symptoms6 often trigger a battery of empiric therapies, such as acid suppression, feeding modifications, and positional changes.7,8,9,10 Prevalence of GERD ranges from 2 to 30% across neonatal intensive care unit (NICU)s in the United States, along with a 13-fold variation in therapies, imposing an additional economic burden of over $70K per admission and 30 hospital days.9,10,11,12
The infant GER questionnaire-revised (I-GERQ-R) is a survey of parental/provider perception of symptom burden thought to be due to GERD, with a 6-point decrease indicating clinical improvement.13 Although prior clinical trials for GERD pharmacotherapy have used symptom-based criteria,14,15,16,17 few have evaluated the effectiveness of a bundled holistic approach, i.e., a combination of pharmacologic-, feeding-, and positional approaches in NICU patients. Improvement of parental perception of symptoms and total GER events with left lateral position and proton pump inhibitor (PPI),8 reduction of GER events with infants in prone or left lateral post-prandially,18 and conservative strategies for 2 weeks showed improvement with I-GERQ-R scores among 1−10 months age.19 We observed that decreased feeding volume and prolonged feeding duration were associated with reduced GER events.20 However, a bundled approach combining targeted acid suppression (limited duration), feeding modifications (volume, position, duration) and postprandial positions has not been rigorously examined in infants with proven esophageal acid reflux index (ARI) severity.
Based on this rationale, we have undertaken this clinical trial to determine the effective therapeutic strategies on the clinically meaningful primary outcomes in infants presenting with GERD symptoms who have qualifying ARI criteria. The objective of this RCT was to examine the short- and long-term clinical outcomes among infants treated concurrently over 4 weeks with PPI with randomly assigned feeding strategy modifications while controlling for gestational maturity (preterm or full-term at birth) and severity of esophageal ARI (3–7%, >7%). Our hypothesis was that the study approach (acid suppression, modified feeding volume, duration, and position) was superior in achieving independent oral feeding or a 6-point reduction in I-GERQ-R vs. the conventional (acid suppression alone) feeding approach.
Patients and methods
Study design and experimental protocol
This is a single-center, single-blinded RCT (Clinicaltrials.gov: NCT02486263) comparing the effectiveness of two feeding strategies combined with the use of a PPI (omeprazole) to manage acid-GERD (GERD Management and Therapy trial (GMT trial)). This study was approved by the Institutional Research Board (IRB) at Nationwide Children’s Hospital, Columbus, OH (IRB # 11-00734). Omeprazole is commonly used off-label in this population within the standard of care.9 Data safety monitoring plan was implemented and monitored quarterly by the Data Safety Monitoring Board (DSMB). Written, signed, and informed parental consent was obtained. Health Insurance Portability & Accountability was followed. Study PI and RN coordinators were available 24/7.
Twenty-four-hour pH-impedance studies were performed6,21,22 (Laborie Medical Technologies, Mississauga, ON, Canada). ARI (duration of esophageal acid exposure, %) was calculated.23 I-GERQ-R symptom score13,16,24 was collected. Demographic and clinical outcomes were managed using research electronic data capture tools (REDCap) tools25 for up to 2 years from subject enrollment.
Participant selection, randomization and allocation
Inclusion criteria were: (a) infants admitted with clinical symptoms of GERD between 34 and 60 weeks’ postmenstrual age, with physician’s intent to treat with acid-suppressive therapy, (b) an intake volume of full enteral feeds ≥150 mL/kg/day, (c) room air or supplemental oxygen ≤1 L per minute, and (d) ARI ≥ 3%.6,21,22,23 Exclusion criteria were: (a) infants with known genetic, metabolic or syndromic diseases; (b) neurological diseases including ≥grade III intraventricular hemorrhage or perinatal asphyxia, (c) GI malformations or surgical GI conditions, and (d) infants on acid-suppressive medication at initial evaluation. Randomization was performed among consented subjects who were stratified 1:1 ratio by ARI severity (3−7%: indeterminate acid reflux and >7%: severe acid reflux) and by birth gestation (preterm, full-term) into study feeding approach or conventional approach. Permuted block randomization with block sizes of 2, 4, 6, and 8 was undertaken by the study statistician (OSU Center for Biostatistics) using a computer-generated allocation and implemented in REDCap. Nurse coordinator enrolled subjects by verifying eligibility, obtaining parental consent, and entering demographic data into REDCap. PI and study staff who evaluated subject clinical outcomes were blinded to study allocation.
Study interventions
Interventions
Providers employ uniform feeding and nutritional practices in our NICU infants as per our standardized guidelines, which applies to nutrient and volume modifications. However, upon randomization and allocation, individual protocols are complied with. Upon completion of screening, enrollment and randomization, the assigned feeding management strategy was relayed to parents and the medical team. Subjects in both arms received omeprazole off-label, as a therapeutic choice26,27 at a recommended dose of 0.75 mg/kg/dose BID.14,26,28 The conventional approach was to not adjust provider-recommended feeding strategies (i.e. fed in any position, duration, volume, and postprandial position). The study approach utilized a modified feeding strategy including: (a) feeding in the right lateral position to facilitate intraprandial gastric emptying,29 (b) feeding duration of at least 30 min utilizing pacing when orally fed to ensure completion of prescribed volumes or via pump to ensure steady delivery of milk if gavage-fed,20 (c) supine postprandial position,30 and (d) limiting total feeding volume to ≤140 mL/kg/day.20
Outcome measures
The a priori primary end-point was achieving independent oral feeds and/or a 6-point decrease in I-GERQ-R score at 5 weeks or sooner, whichever was earliest at discharge. To clarify further, there were two scenarios: (1) Among infants who were transitioning to oral feeds (gavage-fed) at inception: success was defined as achieving full oral feeds or a >6-point decrease from baseline I-GERQ-R. (2) Among infants who were on full oral feeds at inception, success was determined if full oral feeds were maintained plus a ≥6-point decrease from baseline I-GERQ-R. Secondary end-points included growth metrics (weight, length and head circumference), supplemental oxygen, economic metrics (LOHS), long-term feeding outcomes at 6 months and 1 year, and developmental outcomes at 2 years.31,32
Study oversight
Compliance to protocol and data integrity were maintained. Patient care data were stored and secured. Study recruitment criteria were reported to DSMB quarterly and IRB annually. Compliance measurements were documented as intake volumes, feeding durations, feeding positions, postprandial positions and symptom scores, growth metrics and nutritional status. Compliance to administration of omeprazole was confirmed using electronic medical records (Epic, Epic Systems Corporation, Verona, WI, USA) and or parental validation. Trial protocol and important changes to methods after trial commencement are listed in Supplement 1.
Statistical methods
Based on our preliminary data, we had planned to enroll 100 patients (50 per group) to detect 27% or higher increase in proportion of success of the study group compared to the conventional group with 80% power and overall one-sided α level of 0.025. One interim futility analysis was planned at about 50% information prior to the final analysis at 100% information, corresponding to 50 and 100 evaluable patients, respectively. The boundary was determined using Lan-DeMets spending functions to simulate O’Brein−Fleming boundaries.33 Using the target proportion of success, the boundary at the futility analysis was P > 0.297.
Seventy-six infants were randomly assigned till the end of funding for this study and were included in the analysis of demographics and clinical characteristics (Fig. 1, Table 1) and primary outcome by intent-to-treat. If a patient dropped out before the end of study and no symptom score was evaluable, we treated the patient as a failure for the primary outcome by intention-to-treat. Secondary outcome analysis was performed for 72 subjects (Fig. 1). Futility boundary was not reached at interim analysis of 50 patients (P = 0.1 < 0.297) and accrual was continued with DSMB approval. Summary statistics were calculated for patient demographics and clinical characteristics for final analysis. Success rate in achieving PO or reduction in the I-GERQ-R by 6-points was calculated with 95% confidence interval and compared using chi-square test between the conventional and study groups (primary outcomes) for the intention-to-treat and treat-as-treated analyses. Fisher’s exact or chi-square test were used to compare other categorical secondary outcomes including feeding method and supplemental oxygen between the groups. Shapiro−Wilk test for normality was used for the continuous outcomes. Paired t tests or Wilcoxon signed-rank tests were used to assess changes in growth velocity and feeding therapy characteristics between time-1 and time-2. Two sample t tests or Wilcoxon rank-sum tests were used to compare these continuous outcomes between the conventional and study groups. Median (interquartile range (IQR)), mean (SD), or % was reported, unless stated otherwise. P values < 0.05 were considered statistically significant, and SAS version 9.4 (SAS, Inc, an IBM Company, Chicago, IL) was used.
Results
Participant characteristics
Screening, recruitment and follow-up of subjects occurred between August 2012 and October 2018, and data were locked till May 2019. Recruitment ended to allow for clinical outcome analysis. From the 688 infants assessed for eligibility, ARI was: normal (<3%) in 246 (36%), indeterminate (3–7%) in 169 (25%), and abnormal (>7%) in 273 (40%). Study enrollment, randomization, and primary outcome analysis are described in the CONSORT diagram (Fig. 1). Demographic and clinical characteristics at allocation were not significantly different in both groups (Table 1). Frequency (%) of GERD referral reasons were for respiratory concerns (apnea/bradycardia/desaturation, airway management, or suspected aspiration) in 54%, feeding concerns (poor oral feeding or intolerance) in 47%, and GERD-type symptoms (arching/irritability or emesis) in 25% (note proportions do not add to 100 due to providers being able to list multiple reasons for referral). Reasons for referral did not differ between the conventional and study groups (all P > 0.05). Proportion of milk types (exclusive breast milk:exclusive formula:combination of formula and breast milk, %) were not different between groups: 19:67:14 in the conventional vs. 18:53:3 in the study groups (P = 0.24). Of those formula-fed (28 in the conventional group and 29 in the study group), proportion of formula types (hydrolyzed:gentle:low lactose:preterm:standard, %) were: 4:7:4:75:10 in the conventional vs. 10:10:0:69:10 in the study groups (P = 0.84). Caloric density ranged from 19 to 30 cal/oz, and the proportions (%) (19:20:22:24:27:30 cal/oz) for the conventional (11: 31: 33: 19: 6) and the study groups (8:20:30:35:3) did not differ (P = 0.47). Breast milk intake in both groups was 40% at inception (P = 1.0), and the caloric density (cal/oz) for the conventional vs. study groups was 24 21,22,23,24 and 24 21,22,23,24,25,26 respectively (P = 0.41) For the conventional and study groups respectively, acid-suppressive dose (mg/kg/dose BID) was 0.75 [0.75–0.75] vs. 0.75 [0.75–0.75], P = 0.27 upon initial dose, and 0.75 [0.75–1.0] vs. 1.0 [0.75–1.0], P = 0.09 at follow-up.
Study outcomes
Primary and secondary clinical outcomes
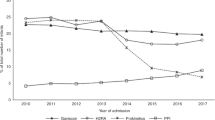
The clinically meaningful primary and secondary outcomes did not differ significantly between the groups (Table 2). I-GERQ-R scores for the study and conventional groups are shown (Fig. 2). At inception: positive I-GERQ-R was 19/35 (54%) in the conventional group vs. 24/37 (65%) in the study group, P = 0.36. At Time-2: positive I-GERQ-R prevalence was 9/31 (29%) in the conventional group vs. 13/34 (38%) in the study group, P = 0.43. In the study group vs. conventional group, respectively: (a) primary outcome achieved in 33% (95% CI, 19−49%) vs. 44% (95% CI, 28−62%) (P = 0.28), (b) secondary outcomes: independent oral feeding in 65% (95% CI, 48−80%) vs. 77% (95% CI, 60−90%), P = 0.26, ≥6-point I-GERQ-R decrease in 38% (95% CI, 22−56%) vs. 35% (95% CI, 19−55%), P = 0.82, length of stay was 98 [81–132] days vs. 108 [83–125] days, P = 0.89, and oxygen requirement at discharge in 19% (95% CI, 8−35%) vs. 26% (95% CI, 13−43%), P = 0.49. There were no significant differences in growth metrics (all P > 0.05) or developmental scores at 2 years (all P > 0.05). Feeding outcomes or I-GERQ-R scores did not significantly differ between the conventional vs. study groups based on feeding method at inception (Table 3). Individual I-GERQ-R questions relating to vomiting, regurgitation, and crying (i.e. frequency of emesis, volume of emesis, symptoms with emesis, and crying more than usual in the past week) had no differences (P > 0.05) within the group or between the groups across maturation for these individual symptoms, except for symptoms with emesis (never:rarely:sometimes:often:always, %) was 16:16:42:19:6 for the conventional group at Time-1 vs. 18:18:29:0:35 for the study group at Time-1, P < 0.01.
Compliance measures, side effects and adverse events
Compliance to randomization, allocation and interventions, and drop outs are reported (Fig. 1, Table 2). Total fluid volume was identical at inception but both groups showed a reduction compared to baseline at Time-2. However, as per the trial design, the study group showed significantly (all P < 0.05) lower volume intake, feeding in right side lying position, and postprandial supine position. Feeding duration of actual feeding was increased in the study group but not statistically different from the conventional group. No side effects or adverse events were reported in either group.
Discussion
In this RCT, while controlling for birth gestation and severity of acidity, we compared the effectiveness of acid suppression with or without a systematic feeding modification bundle in modifying feeding outcomes and I-GERQ-R scores. We found no differences in our a priori primary outcome or preassigned secondary outcomes. Important clinical and research implications can be noted despite the nonsuperiority of the feeding bundle.
Diagnostic conundrums and management issues with GERD in the NICU setting persist. Prior studies8,18,19 used perceived clinical symptoms as a basis for acid suppression, but studies have shown lack of benefit on symptom improvement.34,35 Recent work by us6,20,21 suggests that such symptoms are often due to pharyngo-esophageal provocation or cross-systems activation of reflexes, and can occur during nonacid events or swallowing events, or during transient relaxation of lower esophageal sphincter.29 However, the inability to handle the refluxate determines the “troublesomeness of the symptoms” rather than the esophageal acid exposure. Clinical practice varies when pathophysiological reasoning is not commonly applied. Clinical practices can have unintended consequences1,36,37,38,39 resulting from acid suppression, undernutrition, delays with feeding milestones, decisional conflicts, discharge outcomes and prolonged hospitalization, all of which can escalate burden.9,26
Salient features of our study include (1) allocations were unbiased and appropriately distributed between the groups; (2) among those presenting with GERD symptoms at inception, about 36% of infants had normal esophageal acid exposure, while 40% had abnormal acid exposure, and the rest in the indeterminate range; (3) the study bundle was not superior to acid suppression alone in improving primary outcomes or secondary outcomes; (4) restricted feeding volume, body positions (intra- and postprandial), oral or gavage feeding methods, supplemental oxygen, birth gestation and postnatal maturation did not influence the primary or secondary outcomes; (5) reliability of compliance among those discharged was based on parental trust and available information; (6) there were no reported adverse events. No differences in long-term developmental outcomes or economic burden measures, such as, LOHS, feeding methods and respiratory support at discharge were noted; (7) symptom scores (I-GERQ-R) were significantly lower in both groups, suggesting that maturation may play a role in symptom modification, and not the bundled approaches; (8) feeding outcomes improved in both the groups.
GMT trial strengths and clinical implications are several: (1) Random allocation, study design and protocol adherence were robust and rigorous. Although our strict inclusion criteria may have led to lower eligibility, our approach resulted in identifying infants carefully with true ARI as a marker of esophageal acid exposure. Objective determination of GERD based on ARI > 7% is justifiable in future trials, as nearly 40% of infants are in this severe range, and it is possible to study such a group in larger clinical trials based on pH and impedance criteria, while employing placebo for equipoise. Since time-limited PPI therapy concurrent with feeding strategies was neither shown to be beneficial or associated with adverse effects, we believe that it is safe to include a completely untreated placebo group in future trials that enroll patients with objectively determined acid-GERD. (2) The management strategies were tightly regulated, as were feeding and testing guidelines, and the treatment was uniformly delivered across the two groups. The patient population was homogeneous and constituted a fair representation from the convalescing NICU population. In addition, the randomized controlled allocation accounted for premature or full-term birth, and the indeterminate or determinate acid-GERD per ARI. Furthermore, the prevalence of oxygen requirement or tube feeding at discharge was not different between the groups. (3) Our study trial has many elements of objectivity. Determination of I-GERQ-R and ARI, as well as monitoring feeding methods during the trial are strengths. Thirty-six percent of those with aerodigestive and or cardiorespiratory symptoms perceived by their clinicians to be due to GERD prior to trial consent were not randomized and were also never treated with a PPI as the esophageal acid exposure was normal (ARI < 3%). In a purely symptom-based clinical trial, all those 688 infants screened would have likely been treated for presumed GERD. In the current study, only those that had true ARI exposure have been randomized and treated. Therefore, the symptom-based approach alone is not the solution to diagnose and treat GERD. Interestingly, perception of symptoms (IGERQR scores) decreased across time regardless of treatment group allocations (Fig. 2). These findings strongly support maturational effect. As both the groups were treated with PPI, placebo-included RCTs are needed to determine if maturation alone will improve objectively determined acid- and nonacid-GERD. (4) Absence of pH-impedance testing to confirm true acid-GERD prior to randomization would have resulted in all patients being treated based on subjective, nonspecific symptoms alone. Owing to the strict inclusion and exclusion criteria of this RCT, those with indeterminate and abnormal ARI were treated with a PPI. In the future, a careful RCT that tests the utility of PPI treatment for confirmed acid-GERD by allocating patients to either limited PPI treatment or placebo is indicated to determine whether PPI treatment is needed. The effectiveness of our short-term use of PPI for 4 weeks to improve GERD-attributable symptoms should be tested in future trials. Effect of esophageal acid exposure and therapies on primary mechanistic outcomes of esophageal motility and symptom causation will be addressed in future reports. (5) In routine clinical practice, feeding volumes are modified and alterations in feeding positions are used to manage symptoms. Our study did not show any differences in the outcomes with feeding- and position modifications. Furthermore, volume restriction had no influence on the study outcomes. The improvement in symptoms and feeding outcomes over time irrespective of PPI or feeding modifications may suggest a maturational effect.
It is important to note that major mechanisms of GER, i.e. transient LES relaxation is the major reason for any reflux events—both acid or nonacid substrate. Our therapeutic target was ARI in this study via PPI, feeding volumes, and positional changes. Given that acid GER can also have weakly or nonacid either before or after PPI therapy, and that there were no differences in outcomes between the two groups, we speculate that neither PPI, feeding volume, or positional changes modify the studied indices or symptoms. Maturation under optimal conditions of good nutrition along with placebo-controlled trials are needed to answer the importance of weakly acid or nonacid GER, which would require a multicenter trial with a large group of infants with appropriate physiologic diagnostic testing.
Our study has limitations: (1) Parental and physician biases appeared to be a barrier to recruitment. Recruitment was slow despite the high prevalence of GERD-associated symptoms and a high eligibility rate. This is concerning, as many parents refused clinical trial participation. Many infants did not have true acid-GERD, and fluid restriction often occurred before testing. Interestingly, in some cases, parents and providers did not want to stop the PPI use. These barriers to recruitment can be mitigated in future larger trials with better parent−provider education, as no major effects on the primary or secondary outcomes were noted in our study with or without our allocated bundled GERD treatment. (2) Owing to the higher screening to eligibility ratio, rigorous inclusion criteria, and strict study protocols, we could not complete the recruitment as originally planned of 100 evaluable patients. Seventy-six infants were randomly assigned during the funding period. However, using this cumulative sample size of 72 patients for interim monitoring, we found that we would stop for futility at this time point even if the funding period was not ended. (3) Further studies are needed to correlate parental/provider perception of symptoms (I-GERQ-R) with true symptoms and symptom indices examined during pH-impedance testing. Such studies should also address the severity of acid exposure index in relation to changes in symptom indices.
Conclusion
We addressed the current practice controversies in this clinical trial: (1) Screening and identifying acid-GERD objectively is possible in symptomatic infants prior to any pharmacotherapy. (2) Feeding strategy modification (fluid restriction, positional changes, prolonged feeding duration) has no role in decreasing reflux-type symptoms or in improving the primary outcome of achieving independent oral feeds and/or a 6-point decrease in I-GERQ-R score. (3) No difference in the prevalence of chronic lung disease was noted between the groups. (4) I-GERQ-R scores decreased across time regardless of treatment group allocations that strongly support maturational effect. However, we did not detect an effect on a priori short- or long-term outcomes following randomized allocations. As restrictive feeding strategies do not make a difference, placebo-controlled clinical trials in a larger cohort of convalescing NICU infants with objectively determined newer GERD criteria must be addressed in future trials.
References
Collins, C. R., Hasenstab, K. A., Nawaz, S. & Jadcherla, S. R. Mechanisms of aerodigestive symptoms in infants with varying acid reflux index determined by esophageal manometry. J. Pediatr. 206, 240–247 (2019).
Lopez-Alonso, M. et al. Twenty-four-hour esophageal impedance-pH monitoring in healthy preterm neonates: rate and characteristics of acid, weakly acidic, and weakly alkaline gastroesophageal reflux. Pediatrics 118, e299–e308 (2006).
Rosen, R. et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 66, 516–554 (2018).
Lightdale, J. R., Gremse, D. A. & Section on Gastroenterology Hepatology and Nutrition. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics 131, e1684–e1695 (2013).
Tipnis, N. A. & Tipnis, S. M. Controversies in the treatment of gastroesophageal reflux disease in preterm infants. Clin. Perinatol. 36, 153–164 (2009).
Jadcherla, S. R. et al. Spatiotemporal characteristics of acid refluxate and relationship to symptoms in premature and term infants with chronic lung disease. Am. J. Gastroenterol. 103, 720–728 (2008).
Gonzalez Ayerbe, J. I., Hauser, B., Salvatore, S. & Vandenplas, Y. Diagnosis and management of gastroesophageal reflux disease in infants and children: from guidelines to clinical practice. Pediatr. Gastroenterol. Hepatol. Nutr. 22, 107–121 (2019).
Loots, C. et al. Body positioning and medical therapy for infantile gastroesophageal reflux symptoms. J. Pediatr. Gastroenterol. Nutr. 59, 237–243 (2014).
Jadcherla, S. R. et al. Practice variance, prevalence, and economic burden of premature infants diagnosed with GERD. Hosp. Pediatr. 3, 335–341 (2013).
Slaughter, J. L., Stenger, M. R., Reagan, P. B. & Jadcherla, S. R. Neonatal histamine-2 receptor antagonist and proton pump inhibitor treatment at United States children’s hospitals. J. Pediatr. 174, 63–70.e63 (2016).
Rossor, T., Andradi, G., Bhat, R. & Greenough, A. Investigation and management of gastro-oesophageal reflux in United Kingdom neonatal intensive care units. Acta Paediatr. 107, 48–51 (2018).
Rossor, T. et al. Detection of gastro-oesophageal reflux in the neonatal unit. Acta Paediatr. 107, 1535–1540 (2018).
Kleinman, L. et al. The infant gastroesophageal reflux questionnaire revised: development and validation as an evaluative instrument. Clin. Gastroenterol. Hepatol. 4, 588–596 (2006).
Omari, T. I., Haslam, R. R., Lundborg, P. & Davidson, G. P. Effect of omeprazole on acid gastroesophageal reflux and gastric acidity in preterm infants with pathological acid reflux. J. Pediatr. Gastroenterol. Nutr. 44, 41–44 (2007).
Moore, D. J. et al. Double-blind placebo-controlled trial of omeprazole in irritable infants with gastroesophageal reflux. J. Pediatr. 143, 219–223 (2003).
Orenstein, S. R. et al. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J. Pediatr. 154, 514–520.e514 (2009).
Orenstein, S. R. et al. Famotidine for infant gastro-oesophageal reflux: a multi-centre, randomized, placebo-controlled, withdrawal trial. Aliment. Pharm. Ther. 17, 1097–1107 (2003).
Corvaglia, L. et al. The effect of body positioning on gastroesophageal reflux in premature infants: evaluation by combined impedance and pH monitoring. J. Pediatr. 151, 591–596.e591 (2007).
Orenstein, S. R. & McGowan, J. D. Efficacy of conservative therapy as taught in the primary care setting for symptoms suggesting infant gastroesophageal reflux. J. Pediatr. 152, 310–314 (2008).
Jadcherla, S. R. et al. Impact of feeding strategies on the frequency and clearance of acid and nonacid gastroesophageal reflux events in dysphagic neonates. J. Parenter. Enter. Nutr. 36, 449–455 (2012).
Jadcherla, S. R. et al. Significance of gastroesophageal refluxate in relation to physical, chemical, and spatiotemporal characteristics in symptomatic intensive care unit neonates. Pediatr. Res. 70, 192–198 (2011).
Sivalingam, M. et al. Effects of esophageal acidification on symptoms: an approach to characterize true acid GERD in dysphagic neonates. Dysphagia. 32, 509–519 (2017).
Vandenplas, Y. et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J. Pediatr. Gastroenterol. Nutr. 49, 498–547 (2009).
Orenstein, S. R. Symptoms and reflux in infants: Infant Gastroesophageal Reflux Questionnaire Revised (I-GERQ-R)-utility for symptom tracking and diagnosis. Curr. Gastroenterol. Rep. 12, 431–436 (2010).
Harris, P. A. et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 42, 377–381 (2009).
Gunasekaran, T. S. & Hassall, E. G. Efficacy and safety of omeprazole for severe gastroesophageal reflux in children. J. Pediatr. 123, 148–154 (1993).
Bishop, J., Furman, M. & Thomson, M. Omeprazole for gastroesophageal reflux disease in the first 2 years of life: a dose-finding study with dual-channel pH monitoring. J. Pediatr. Gastroenterol. Nutr. 45, 50–55 (2007).
Kaguelidou, F. et al. Dose-finding study of omeprazole on gastric pH in neonates with gastro-esophageal acid reflux using a Bayesian sequential approach. PLoS ONE 11, e0166207 (2016).
Omari, T. I. et al. Paradoxical impact of body positioning on gastroesophageal reflux and gastric emptying in the premature neonate. J. Pediatr. 145, 194–200 (2004).
Hauk, L. SIDS and safe sleeping environments for infants: AAP updates recommendations. Am. Fam. Physician 95, 806–807 (2017).
Adams-Chapman, I. et al. Association between feeding difficulties and language delay in preterm infants using Bayley Scales of Infant Development-Third Edition. J. Pediatr. 163, 680–685.e681−e683 (2013).
Jadcherla, S. R. et al. Feeding methods at discharge predict long-term feeding and neurodevelopmental outcomes in preterm infants referred for gastrostomy evaluation. J. Pediatr. 181, 125–130 e121 (2017).
Lan, K. K. G. & Demets, D. L. Discrete sequential boundaries for clinical-trials. Biometrika 70, 659–663 (1983).
Tighe, M. et al. Pharmacological treatment of children with gastro-oesophageal reflux. Cochrane Database Syst. Rev. 11, CD008550 (2014).
Cieruszczak-Bialek, D. et al. No effect of proton pump inhibitors on crying and irritability in infants: systematic review of randomized controlled trials. J. Pediatr. 166, 767–770.e763 (2015).
Bell, J. C. et al. Acid suppressants for managing gastro-oesophageal reflux and gastro-oesophageal reflux disease in infants: a national survey. Arch. Dis. Child 103, 660–664. (2018).
Malchodi, L. et al. Early acid suppression therapy exposure and fracture in young children. Pediatrics 144, e20182625 (2019).
Gold, B. D. Is gastroesophageal reflux disease really a life-long disease: do babies who regurgitate grow up to be adults with GERD complications? Am. J. Gastroenterol. 101, 641–644 (2006).
Malcolm, W. F. & Cotten, C. M. Metoclopramide, H2 blockers, and proton pump inhibitors: pharmacotherapy for gastroesophageal reflux in neonates. Clin. Perinatol. 39, 99–109 (2012).
Acknowledgements
The authors would like to thank the infants and their parents who participated in this study; Rebecca Moore, MACPR, BSN, RN, for clinical support; and the Data Safety Monitoring Board, IRB, and Audit departments at the Abigail Wexner Research Institute at Nationwide Children’s Hospital, Columbus, OH, USA. This work was supported by the National Institutes of Health (RO1 DK 068158 (to S.R.J.)) and the National Center for Advancing Translational Sciences (UL1TR002733 (to The Ohio State University Center for Clinical and Translational Science for REDCap support)).
Author information
Authors and Affiliations
Contributions
S.R.J. obtained funding, conceptualized and designed the study, performed procedures and data acquisition, supervised data analysis, interpreted data, drafted the initial manuscript, and reviewed and revised the manuscript. K.A.H. performed data acquisition, analysis, and interpretation of data, drafted the initial manuscript, and reviewed and revised the manuscript. L.W. designed the study, performed statistical analysis, interpreted data, and reviewed and revised the manuscript. E.K.O. performed data acquisition, analysis, and interpretation of data, and reviewed and revised the manuscript. S.V. and I.K.G. performed procedures and data acquisition, interpretation of data, and reviewed and revised the manuscript. J.L.S. and C.D.L. provided additional intellectual input, interpreted data, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent patient
Consent was required, and informed parental consent was obtained prior to study participation and the study was approved by the Abigail Wexner Research Institute at Nationwide Children’s Hospital.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jadcherla, S.R., Hasenstab, K.A., Wei, L. et al. Role of feeding strategy bundle with acid-suppressive therapy in infants with esophageal acid reflux exposure: a randomized controlled trial. Pediatr Res 89, 645–652 (2021). https://doi.org/10.1038/s41390-020-0932-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0932-4
This article is cited by
-
Characteristics of esophageal refluxate and symptoms in infants compared between pre-treatment and on treatment with proton pump inhibitors
Journal of Perinatology (2024)
-
A Review of Histamine-2 Receptor Antagonist and Proton Pump Inhibitor Therapy for Gastroesophageal Reflux Disease in Neonates and Infants
Pediatric Drugs (2023)
-
Anti-reflux medication use in preterm infants
Pediatric Research (2022)
-
A quality improvement initiative to reduce acid-suppressing medication exposure in the NICU
Journal of Perinatology (2022)