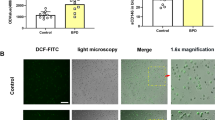
Abstract
Background
Intrauterine inflammation affects fetal lung development. BTB and CNC homology 1 (Bach1) is a transcriptional repressor of heme oxygenase-1 (HO-1) and interleukin-6 (IL-6) genes. We investigated the role of Bach1 in the development of fetal mouse lungs exposed to lipopolysaccharide (LPS) using a whole fetal lung tissue culture system.
Methods
We isolated and cultured embryonic day 12.5 fetal mouse lungs from pregnant Bach1 knockout (−/−) and wild-type (WT) mice. Airway branching morphogenesis was assessed by microscopically counting peripheral lung buds after incubation with/without LPS. Expression levels of genes related to inflammation and oxidative stress were evaluated using quantitative PCR. Zinc protoporphyrin, HO-1-specific inhibitor, was used.
Results
Branching morphogenesis was observed in Bach1−/− and WT fetal mice lungs without LPS exposure; after exposure to LPS, the number of peripheral lung buds was suppressed in Bach1−/− group only. Basal messenger RNA (mRNA) and protein expression of HO-1 was significantly higher in Bach1−/− group than in WT group; IL-6 and monocyte chemoattractant protein-1 mRNA expression was significantly increased after LPS exposure in both groups. Zinc protoporphyrin mitigated the LPS-induced suppression of branching morphogenesis in Bach1−/− mice.
Conclusion
The ablation of Bach1 suppresses airway branching morphogenesis after LPS exposure by increased basal expression levels of HO-1.
Similar content being viewed by others
Introduction
During fetal lung morphogenesis, simple epithelial tubes develop into a complex structure to enable adequate gas exchange post birth. Normally during these stages, small terminal airways branch, expand, and divide to form alveolar ducts.1 This process fails to occur in extremely preterm infants with bronchopulmonary dysplasia (BPD), which is known as chronic lung disease of prematurity. BPD results from arrested airway morphogenesis during the canalicular and saccular stages of lung development.2,3,4 In BPD patients, arrested lung development leads to fewer airways and alveoli, and reduced capacity for gas exchange. Various environmental factors, including either airway epithelial injury or lung inflammation, are implicated in development of BPD.1,5,6
Intrauterine inflammation, which most commonly presents as chorioamnionitis (CAM), is one of the leading causes of BPD.5,7 In animal models, intrauterine inflammation induced by intraamniotic injection of interleukin-1α (IL-1α), IL-1β, or lipopolysaccharide (LPS) can cause structural changes in the fetal animal lung and affect the expression of growth factors that are essential for airway branching morphogenesis. These alterations cause alveolar and microvascular simplification resembling the histology of BPD.4 In ex vivo models, LPS can inhibit airway branching morphogenesis in fetal lung explants in the absence of circulating inflammatory cells, suggesting that rodent lung cells are competent to transduce signals that interrupt normal lung development.3,6 Multiple gene targets of inflammatory signaling that are critical for normal airway morphogenesis are identified.7,8
BTB and CNC homology 1 (Bach1) is a transcriptional repressor protein of heme oxygenase-1 (HO-1), β-globin, and IL-6 genes.9,10,11 In Bach1 knockout (−/−) mice, the transcription of HO-1 is constitutively upregulated, leading to increased HO-1 protein levels and enzymatic activity under normal conditions in several organs, including the heart, lungs, and liver.11,12 Previous studies showed that Bach1−/− adult mice are protected from conditions caused by oxidative stress, including atherosclerosis, myocardial ischemia/reperfusion injury, and hyperoxic lung injury.11,13 The mechanism underlying the protection of Bach1−/− mice from conditions caused by oxidative stress can be explained, in part, by the upregulation of HO-1 activity;14 however, much remains unelucidated.
We previously demonstrated, in an animal models of BPD, that Bach1−/− neonatal mice are well recovered from hyperoxia-induced lung injury during the recovery phase compared with wild-type (WT) mice via antioxidant/anti-inflammatory activity of HO-1 and/or the transient overexpression of proinflammatory cytokines, such as IL-6 and monocyte chemoattractant protein-1 (MCP-1).13 Therefore, we hypothesized that Bach1−/− fetal mouse lungs would show tolerance against inflammation and oxidative stress. In the present study, our objective was to investigate the role of Bach1 gene in the development of fetal mouse lungs exposed to LPS using a whole fetal lung tissue culture system.
Methods
Animals
All procedures and protocols were approved by the animal Care and Use Committee of Saitama Medical University, Japan (permit number: 1701). C57BL/6 WT mice were purchased from Nippon SLC, Inc. (Hamamatsu, Shizuoka, Japan) and fed in the animal facility at Saitama Medical Center, Saitama Medical University. Bach1−/− mice, originally produced by Sun et al.14 and repeatedly backcrossed with C57BL/6 mice for at least 12 generations, were maintained in the animal facility at our center. Genotyping was performed using PCR analysis of tail biopsies.
Explant culture of mouse embryonic whole lung exposed to LPS
Embryonic day (E) 12.5 fetal lungs were isolated from pregnant Bach1−/− or WT mice. All procedures were performed under sterile conditions and the isolated lung tissues were washed in 1× Hank’s balanced salt solution (Gibco-BRL, Grand Island, NY, USA) supplemented with 50 U/mL penicillin–streptomycin (PS; Sigma-Aldrich, St. Louis, MO). Explant culture of mice embryonic whole lung was performed according to the modified protocol described by del Moral and Warburton.15 Briefly, the lung tissue was placed on a polycarbonate membrane filter (Toyo Roshi, Kaisha, Ltd, Tokyo, Japan) in Dulbecco’s modified Eagle’s medium (Gibco-BRL) supplemented with 10% fetal calf serum (Gibco-BRL) and 50 U/mL of PS, with or without 100 or 500 μg/mL LPS from Escherichia coli, serotype O55:B5 (Sigma-Aldrich), and grown at 37 °C, in a 95% air/5% CO2, and humidified atmosphere (Fig. 1a).
Experimental scheme. a Whole fetal lungs (E12.5) derived from WT and Bach1−/− mouse were explanted and cultured supplemented with or without 100 or 500 μg/mL LPS from E. coli, serotype O55:B5 and grown at a 37 °C, 95% air/5% CO2, and humidified atmosphere. b ZnPP (100 pg/mL), a chemical inhibitor of HO-1 enzyme activity, was added and cultured in WT and Bach1−/− fetal mouse lung with or without 100 μg/mL LPS. LPS, lipopolysaccharide; ZnPP, zinc protoporphyrin
Inhibition of HO-1 by zinc protoporphyrin IX
For inhibition of HO-1 enzymatic activity, the fetal lung tissues were cultured in the medium with 100 pg/mL of zinc protoporphyrin IX (ZnPP; Santa Cruz Biotechnology Inc., Dallas, TX) in 0.4% dimethyl sulfoxide (Fujifilm Wako Chemicals Europe GmbH, Fuggerstrasse, Germany) from E12.5 (Fig. 1b).
Quantification of airway branching morphogenesis
Bach1−/− and WT fetal mice lung tissues with or without LPS exposure (either 100 or 500 µg/mL) were observed every 24 h for up to 96 h (Fig. 1a). Airway branching morphogenesis was assessed by counting the number of peripheral lung buds visible around the periphery of the lung explants under an inverted light microscope (CKX41 Inverted Microscope, Olympus, Tokyo, Japan). The data were reported as the fold change in the number of peripheral lung buds for each time point relative to those counted on E12.5. Five lungs were counted at each time point.
RNA extraction and qPCR analysis
The messenger RNA (mRNA) expression levels of HO-1, IL-6, MCP-1, IL-1β, tumor necrosis factor (TNF), nuclear factor erythroid 2-related factor 2 (Nfe2l2), transformation related protein 53 (Trp53), and fibroblast growth factor (FGF)-10 in Bach1−/− and WT fetal mice lung tissues after 24 h of explant culture with or without LPS exposure (100 µg/mL) were measured using quantitative PCR (qPCR). Five samples from each group were used. The tissue samples were homogenized by passing them several times through a 23-gauge needle fitted to a syringe. Total RNA was isolated and cleaned using the TRIzol method (Gibco-BRL) and RNeasy Mini Kit (Qiagen, Valencia, CA), respectively, according to the manufacturer’s protocols. Total RNA (500 ng) was reverse transcribed (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems, Foster City, CA) and complementary DNA (cDNA) was used in each PCR reaction with primers for HO-1 (Mm00516005), IL-6 (Mm00446190), MCP-1 (Mm00441242), TNF (Mm00443258_m1), IL-1β (Mm00434228_m1), Nfe2l2 (Mm00477784_m1), Trp53 (Mm01731290_g1), and FGF-10 (Mm00433275_m1), and TaqMan Universal PCR Master Mix (Applied Biosystems). Analysis was performed using the 7500 Fast Real-Time PCR System (Applied Biosystems). Relative expression was determined using comparative critical threshold method, normalizing each sample to β-glucuronidase (Mm01197698).
Evaluation of HO-1 protein levels in lung homogenates
Western analysis was performed to evaluate HO-1 protein levels. The blotted membranes were exposed to anti-HO-1 (1:2000; ab13243, Abcam, Cambridgeshire, UK) or anti-β-actin (1:5000; GTX26276, GeneTex, Irvine, CA), followed by horseradish peroxidase-labeled secondary antibodies. Chemiluminescence (Amersham ECL Prime Western blotting Detection Reagent; GE Healthcare, Little Chalfont, UK) was detected using a digital imaging system (Bio-Rad ChemiDoc XRS+, Bio-Rad, Hercules, CA).
Statistical analysis
Values are expressed as the mean ± standard deviation. For comparison between treatment groups, the null hypothesis that there is no difference between the treatment means was tested by unpaired t tests for two treatment groups. P values <0.05 were considered to be significant.
Results
LPS exposure suppressed airway branching morphogenesis in the WT fetal mouse lungs dose dependently
Under normal condition, well-organized peripheral lung buds were branching in the WT fetal mouse lungs for 96 h (Fig. 2a). After 72 h incubation with 100 or 500 µg/mL of LPS, LPS exposure suppressed the increase in the number of peripheral lung buds in the WT fetal mouse lungs in dose-dependent manner (p < 0.05 at 72 and 96 h after exposure to 500 µg/mL of LPS) (Fig. 2b).
Serial microscopic findings. a The number of peripheral buds was increased by day. Scale bar 300 μm. b Fold increase in branching compared to 0 h (n = 5, each group). LPS exposure significantly suppressed airway branching morphogenesis in the WT fetal mouse lungs supplemented with 500 µg/mL of LPS. *P < 0.05. LPS, lipopolysaccharide
LPS exposure (100 µg/mL) suppressed airway branching morphogenesis in the Bach1 −/− fetal mouse lungs
Peripheral lung buds were coordinately branching under normal condition without LPS in both WT and Bach1−/− fetal mice lungs (Fig. 3a). Exposure to 500 µg/mL of LPS caused a suppression of branching morphogenesis in both WT and Bach1−/− fetal mice lungs from 72 to 96 h after LPS administration (Fig. 3b). Because no significant differences were observed in the number of peripheral lung buds between WT and Bach1−/− fetal mice lungs after exposure to LPS (500 µg/mL), LPS (100 µg/mL) was used for further analysis.
Serial microscopic findings. a The number of peripheral buds was increased by day. Scale bar 300 μm. LPS−, no LPS; LPS+, supplemented with LPS. b, c Fold increase in branching compared to 0 h (n = 5, each group). b LPS exposure (500 µg/mL) significantly suppressed airway branching morphogenesis both in the WT and Bach1−/− fetal mouse lungs. −, no LPS added; +, supplemented with 500 µg/mL LPS. *P < 0.05. c LPS exposure (100 µg/mL) significantly suppressed airway branching morphogenesis in the Bach1−/− fetal mouse lungs. −, no LPS added; +, supplemented with 100 µg/mL LPS. *P < 0.05; **p < 0.01. WT, wild-type; KO, knockout; LPS, lipopolysaccharide
Exposure to 100 µg/mL of LPS significantly suppressed the increase in the number of peripheral lung buds in Bach1−/− fetal mouse lungs at each time point (p < 0.01 at 24, 72, and 96 h after LPS exposure; p < 0.05 at 48 h after LPS exposure); however, this suppression of airway branching morphogenesis was not observed in the WT fetal mouse lungs after LPS exposure (Fig. 3c).
HO-1 mRNA expression levels in Bach1 −/− fetal mouse lungs were significantly higher than those in WT fetal mouse lungs
Because Bach1 is reported to be a transcriptional repressor of the HO-1 and IL-6 genes,11 and the overexpression of HO-1 and proinflammatory cytokines, such as IL-6 and MCP-1, are strongly induced in the Bach1−/− newborn mouse lungs exposed to hyperoxia,13 lung mRNA expression levels of HO-1, IL-6, and MCP-1 were determined using qPCR. Measurements of lung IL-1β, TNF, FGF-10, Nfe2l2, and Trp53 mRNA expression levels were also added, because these are key genes involved in LPS-induced alterations in airway branching morphogenesis16,17,18 and induction of reactive oxygen species.19 Lung IL-6, MCP-1, IL-1β, and TNF mRNA expression levels were significantly greater in WT and Bach1−/− fetal mice after LPS exposure, compared with non-LPS controls (p < 0.01; Fig. 4a, b, d, e). LPS exposure did not alter lung FGF-10 and Trp53 mRNA expression levels in both WT and Bach1−/− mice (Fig. 4f, h). HO-1 mRNA expression levels were significantly greater in the WT fetal mouse lungs after LPS exposure, compared with non-LPS controls (p < 0.01). Bach1−/− mice had consistently enhanced mRNA expression levels of HO-1 both with and without LPS exposure, compared with WT mice (p < 0.05 and p < 0.01, respectively; Fig. 4c).
The mRNA expression levels. Relative mRNA levels are represented as fold-change relative to WT mouse lungs without LPS exposure (100 µg/mL). a IL-6 mRNA expression levels were significantly increased after LPS exposure in both the Bach1−/− and WT fetal mouse lungs. **P < 0.01. b MCP-1 mRNA expression levels were significantly increased after LPS exposure in both the Bach1−/− and WT fetal mouse lungs. **P < 0.01. c HO-1 mRNA expression levels were consistently increased both with and without LPS exposure in the Bach1−/− fetal mouse lungs compared with those in the WT. * P < 0.05; **p < 0.01. d IL-1β mRNA expression levels were significantly increased after LPS exposure in both the Bach1−/− and WT fetal mouse lungs. IL-1β mRNA expression levels were decreased with LPS exposure in the Bach1−/− fetal mouse lungs compared with those in the WT. **P < 0.01. e TNF mRNA expression levels were significantly increased after LPS exposure in both the Bach1−/− and WT fetal mouse lungs. **P < 0.01. f FGF-10 mRNA expression levels were not altered after LPS exposure in both the Bach1−/− and WT fetal mouse lungs. g Nfe2l2 mRNA expression levels were significantly increased after LPS exposure in the WT fetal mouse lungs. *P < 0.05. h Trp53 mRNA expression levels were not altered after LPS exposure in both the Bach1−/− and WT fetal mouse lungs. IL, interleukin; MCP, monocyte chemoattractant protein; HO, heme oxygenase; TNF, tumor necrosis factor; FGF, fibroblast growth factors; Nfe2l2; nuclear factor erythroid 2-related factor 2; Trp53, transformation-related protein 53; WT, wild-type; KO, knockout; LPS, lipopolysaccharide
HO-1 protein expression levels in Bach1 −/− fetal mouse lungs were higher than those in WT fetal mouse lungs
Lung HO-1 protein expression levels were measured using western blot analysis. In the same manner as mRNA expression levels, HO-1 protein levels in Bach1−/− fetal mouse lungs were higher than those in WT fetal mouse lungs after a 24-h incubation both with and without 100 µg/mL LPS exposure (Fig. 5).
HO-1 protein expression levels in the fetal mouse lungs. The WT or Bach1−/−fetal mouse lungs were incubated with or without 100 µg/mL LPS for 24 h. After treatment, HO-1 protein expression levels were analyzed by the Western blotting. HO-1 protein expression levels in the Bach1−/− fetal mouse lungs were higher than those in the WT lungs both in control conditions (no LPS) and after LPS exposure. HO, heme oxygenase; WT, wild-type; KO, knockout; LPS, lipopolysaccharide
ZnPP ameliorated LPS-induced airway branching morphogenesis suppression in Bach1 −/− fetal mouse lungs
To determine the association between HO-1- and LPS-induced airway branching morphogenesis suppression in Bach1−/− fetal mouse lungs, ZnPP, which is an HO-1 inhibitor, or vehicle control was administered to the medium immediately at the onset of LPS exposure on E12.5, and airway branching morphogenesis was analyzed during LPS exposure (Fig. 1b). Administration of ZnPP significantly improved the number of peripheral lung buds in Bach1−/− mice after LPS exposure in comparison with that in mice administered vehicle control (p < 0.05; Fig. 6). Administration of ZnPP alone did not affect airway branching morphogenesis in the lung, which was not exposed to LPS.
Fold increase in branching at 96 h compared to 0 h after incubation in the Bach1−/− fetal mouse lungs. LPS addition significantly suppressed the Bach1−/− fetal mouse lung branching. At 96 h after LPS exposure, the ZnPP administration significantly recovered the decreased number of peripheral lung buds in the Bach1−/− fetal mouse lungs exposed to LPS, compared to no ZnPP administration. LPS−, no LPS added; LPS+, 100 μg/mL LPS added; ZnPP−, no ZnPP added; ZnPP+, 100 pg/mL ZnPP added. *P < 0.05. LPS, lipopolysaccharide; ZnPP, Zinc protoporphyrin
Discussion
This study unexpectedly revealed that fetal mouse lungs lacking Bach1 were vulnerable to LPS-induced delayed fetal lung development. This vulnerability of the Bach1−/− fetal mouse lungs was demonstrated by significantly reduced number of peripheral lung buds found during a morphological examination in comparison with that of the WT mice. Although transcriptional response of HO-1 to LPS exposure was not observed in Bach1−/− fetal mouse lungs, the mRNA expression levels of HO-1, but not those of IL-6, MCP-1, IL-1β, TNF, or FGF-10, were consistently higher both with and without LPS exposure in Bach1−/− lungs in comparison with those in the WT fetal mouse lungs. The increased protein levels of HO-1 were also observed in Bach1−/− fetal mouse lungs both at baseline and after LPS exposure compared with WT fetal mouse lungs. In addition, the administration of an HO-1 inhibitor improved the number of peripheral lung buds in Bach1−/− fetal mouse lungs during LPS exposure, suggesting that Bach1 is a transcriptional repressor of HO-1 and persistently increased mRNA and protein expression levels of HO-1 in Bach1−/− fetal mouse lungs may lead to suppression of fetal airway branching morphogenesis during LPS exposure.
In our previous in vivo study using Bach1−/− newborn mice, they were recovered from hyperoxia-induced lung injury compared with WT mice. This recovery might be due to the antioxidant/anti-inflammatory activity of HO-1 and/or the overexpression of proinflammatory cytokines, such as IL-6 and MCP-1, which were strongly induced in the Bach1−/− mouse lungs.13 In the current study, no differences in the mRNA expression levels of IL-6 and MCP-1 were observed between WT and Bach1−/− fetal mice lungs exposed to LPS. This finding was likely achieved by the different types of exposure (hyperoxia or LPS) or oversized impact of LPS on the expression levels of proinflammatory cytokines.
In this study, whole lungs derived from E12.5 fetal mice were explanted to tissue culture with or without LPS. Of the stages of lung development, the pseudoglandular stage spans from E12 to E16.5 in the mouse and from E35 to E119 (5–17 weeks) in humans. During the pseudoglandular stage in humans, approximately the first 20 generations of future airways are formed and the first few generations of alveolar ducts are laid down by the end of this stage.20 Exposure of these immature lungs to intrauterine infection/inflammation is believed to lead to an impaired lung development, which characterizes BPD. Therefore, our current model parallels the period of lung development occurring prenatally in future extremely preterm infants, making it relevant for investigating the pathogenesis of BPD, especially that which is related to intrauterine infection/inflammation, such as CAM.
Using these ex vivo models to investigate the effects of LPS on embryonic lung branching morphogenesis, previous studies showed that macrophages in the fetal mouse lung mediated the inflammatory response to LPS and that macrophage activation inhibited airway morphogenesis by increased levels of TNF, IL-6, and IL-1β levels and inhibited expression of multiple genes critical for normal lung development, including FGF-10, due to activation of nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling.16,17,18 In the present study, compared with the WT lungs, no increased levels of TNF, IL-6, and IL-1β and no decreased levels of FGF-10 were observed in altered branching airways of Bach1−/− mice after LPS exposure, suggesting that aside from NF-κB and MAPK signaling, Bach1-HO-1 axis also might play an important role in branching morphogenesis and that controlling adequate expression and enzymatic activity levels of HO-1 might be critical during fetal lung development.
HO-1 is a subtype of HO catalyzing the first and rate-limiting enzyme in degradation of heme to biliverdin. Final metabolites, working together with biliverdin reductase, include bilirubin, ferrous iron, and carbon monoxide.21 HO-1 is activated by various oxidative and proinflammatory stressors in a wide variety of mammalian cells and plays a role in cellular anti-inflammatory status, known as a potential antioxidant action.22,23 Increased HO-1 expression was demonstrated in several inflammatory states, such as atherosclerosis, diabetes, sepsis, ischemia–reperfusion injury, organ failure, and organ transplantation.24 In the lung, overexpression of HO-1 in human pulmonary epithelial cells was reported to provide potent cytoprotecitve effects resulting in increased resistance to hyperoxia and tissue injury.25 Furthermore, lung-specific neonatal HO-1 transgenic mice demonstrated vasculoprotective effects under hyperoxia.26 However, Suttner and Dennery27 reported that the effects of HO-1 against hyperoxia changed paradoxically from cytoprotection to cytotoxicity, depending on its concentration, which was added to the cultured cells. Dong et al.28 commented that a wide range of HO-1 inductions have been documented in diverse pathophysiological conditions that are potentially injurious. Furthermore, we previously demonstrated that despite beneficial effects of HO-1 at low levels of expression, there was a reversal of cytoprotection with increased HO-1 expression both in vitro27 and in vivo,29 indicating a beneficial threshold of HO-1 overexpression. In this present study, contrary to our hypothesis, HO-1 overexpression in Bach1−/− fetal mouse lungs did not show protective effects against LPS exposure, supporting the notion that there is a beneficial threshold of HO-1 overexpression in fetal lungs against LPS exposure.
In this study, fetal mouse lungs were treated with two doses (100 and 500 µg/mL) of LPS. Interestingly, no differences in suppressed branching morphogenesis were observed between WT and Bach1−/− fetal mice lungs exposed to high-dose LPS, whereas low-dose LPS suppressed the increase in the number of peripheral lung buds only in Bach1−/− mice. A probable explanation for the differences in responses to the two doses of LPS between WT and KO might be that 500 µg/mL might result in masking of the negative effects of increased HO-1 on lung branching morphogenesis. Meanwhile, in culture medium preparations, differences in their ratios of serum might affect responses to different doses of LPS, as it was previously reported that higher bovine serum albumin preparations enhanced cytokine production by murine macrophages in vitro.30
There are several limitations of this study pointing to areas of future investigations. First, we used explant culture of mouse embryonic whole lung, which was ex vivo model, and, therefore, was not directory transferable to the clinical setting because of the absence of respiratory excursion, subject motions, and the effect of blood flow, and also because of the optimal LPS dose determination for ourselves. Second, despite of the use of HO-1 inhibitor, ZnPP, HO enzymatic activity could not be measured because of the lack of methods suitable for small volume tissue sample, such as an embryonic mouse lung.
In conclusion, Bach1−/− fetal mouse lungs were vulnerable to suppression of airway branching morphogenesis by LPS exposure. This vulnerability seemed to be mediated by the consistent overexpression of HO-1 in Bach1−/− mice after LPS exposure. The involvement of HO-1 in the vulnerability to LPS-induced delayed fetal lung development was confirmed by the experiment in which an HO-1 inhibitor was administered to Bach1−/− fetal mouse lungs in a tissue culture system. Based on these results, we therefore conclude that Bach1 is a transcriptional repressor of HO-1; thus, persistently increased HO-1 in Bach1−/− fetal mouse lungs may lead to a delayed lung development during LPS exposure.
Change history
12 June 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41390-023-02663-6
References
Coalson, J. J. Pathology of new bronchopulmonary dysplasia. Semin. Neonatol. 8, 73–81 (2003).
Baraldi, E. & Filippone, M. Chronic lung disease after premature birth. N. Engl. J. Med. 357, 1946–1955 (2007).
Choi, C. W. et al. Bronchopulmonary dysplasia in a rat model induced by intra-amniotic inflammation and postnatal hyperoxia: morphometric aspects. Pediatr. Res. 65, 323–327 (2009).
Jobe, A. H. et al. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am. J. Obstet. Gynecol. 182, 401–408 (2000).
Jobe, A. H. & Ikegami, M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir. Res. 2, 27–32 (2001).
Vayrynen, O., Glumoff, V. & Hallman, M. Regulation of surfactant proteins by LPS and proinflammatory cytokines in fetal and newborn lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L803–L810 (2002).
Kramer, B. W., Kallapur, S., Newnham, J. & Jobe, A. H. Prenatal inflammation and lung development. Semin. Fetal Neonatal Med. 14, 2–7 (2009).
Shi, W., Zhao, J., Anderson, K. D. & Warburton, D. Gremlin negatively modulates BMP-4 induction of embryonic mouse lung branching morphogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L1030–L1039 (2001).
Maines, M. D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 2, 2557–2568 (1988).
Otterbein, L. E. & Choi, A. M. Heme oxygenase: colors of defense against cellular stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L1029–L1037 (2000).
Tanimoto, T. et al. Genetic ablation of the Bach1 gene reduces hyperoxic lung injury in mice: role of IL-6. Free Radic. Biol. Med. 46, 1119–1126 (2009).
Kassovska-Bratinova, S., Yang, G., Igarashi, K. & Dennery, P. A. Bach1 modulates heme oxygenase-1 expression in the neonatal mouse lung. Pediatr. Res. 65, 145–149 (2009).
Ito, M. et al. Genetic ablation of Bach1 gene enhances recovery from hyperoxic lung injury in newborn mice via transient upregulation of inflammatory genes. Pediatr. Res. 81, 926–931 (2017).
Sun, J. et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 21, 5216–5224 (2002).
Del Moral, P. M. & Warburton, D. Explant culture of mouse embryonic whole lung, isolated epithelium, or mesenchyme under chemically defined conditions as a system to evaluate the molecular mechanism of branching morphogenesis and cellular differentiation. Methods Mol. Biol. 633, 71–79 (2010).
Blackwell, T. S. et al. NF-kappaB signaling in fetal lung macrophages disrupts airway morphogenesis. J. Immunol. 187, 2740–2747 (2011).
Carver, B. J., Plosa, E. J., Stinnett, A. M., Blackwell, T. S. & Prince, L. S. Interactions between NF-kappaB and SP3 connect inflammatory signaling with reduced FGF-10 expression. J. Biol. Chem. 288, 15318–15325 (2013).
Zhao, D., Zhuang, N., Ding, Y., Kang, Y. & Shi, L. MiR-221 activates the NF-kappaB pathway by targeting A20. Biochem. Biophys. Res. Commun. 472, 11–18 (2016).
Han, E. S. et al. The in vivo gene expression signature of oxidative stress. Physiol. Genomics 34, 112–126 (2008).
Kitaoka, H., Burri, P. H. & Weibel, E. R. Development of the human fetal airway tree: analysis of the numerical density of airway endtips. Anat. Rec. 244, 207–213 (1996).
Tenhunen, R., Marver, H. S. & Schmid, R. Microsomal heme oxygenase. Characterization of the enzyme. J. Biol. Chem. 244, 6388–6394 (1969).
Applegate, L. A., Luscher, P. & Tyrrell, R. M. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 51, 974–978 (1991).
Yet, S. F. et al. Induction of heme oxygenase-1 expression in vascular smooth muscle cells. A link to endotoxic shock. J. Biol. Chem. 272, 4295–4301 (1997).
Wagener, F. A. et al. Different faces of the heme-heme oxygenase system in inflammation. Pharm. Rev. 55, 551–571 (2003).
Lee, P. J., Alam, J., Wiegand, G. W. & Choi, A. M. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc. Natl. Acad. Sci. USA 93, 10393–10398 (1996).
Fernandez-Gonzalez, A., Alex Mitsialis, S., Liu, X. & Kourembanas, S. Vasculoprotective effects of heme oxygenase-1 in a murine model of hyperoxia-induced bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L775–L784 (2012).
Suttner, D. M. & Dennery, P. A. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 13, 1800–1809 (1999).
Dong, Z., Lavrovsky, Y., Venkatachalam, M. A. & Roy, A. K. Heme oxygenase-1 in tissue pathology: the Yin and Yang. Am. J. Pathol. 156, 1485–1488 (2000).
Namba, F., Go, H. & Murphy, J. A. Expression level and subcellular localization of heme oxygenase-1 modulates its cytoprotective properties in response to lung injury: a mouse model. PLoS ONE 9, e90936 (2014).
Zheng, Z. M., Specter, S. C. & Lancz, G. Bovine serum albumin preparations enhance in vitro production of tumor necrosis factor alpha by murine macrophages. Immunol. Invest. 24, 737–756 (1995).
Acknowledgements
No financial assistance was received in support of the study.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design (Y.A., K.I., and F.N.), acquisition of data (Y.A., M.I., K.T., J.O., Y.M., and K.M.), or analysis and interpretation of data (Y.A. and F.N.); drafting the article or revising it critically for important intellectual content (Y.A. and F.N.); and final approval of the version to be published (Y.A., M.I., K.T., J.O., Y.M., K.M., K.I., and F.N.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arai, Y., Ito, M., Tanaka, K. et al. Increased expression of heme oxygenase-1 suppresses airway branching morphogenesis in fetal mouse lungs exposed to inflammation. Pediatr Res 87, 494–500 (2020). https://doi.org/10.1038/s41390-019-0588-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0588-0