Abstract
Aim
To investigate the effect of kangaroo care (KC) and its duration on neurobehavioral performance, stress response, breastfeeding success, and vital signs in premature infants.
Methods
One hundred and twenty premature infants were randomized to receive either KC for 60 min daily, KC for 120 min daily or conventional care (controls) for at least 7 days. Salivary cortisol was measured before and after the first KC session and then after 7 days. Temperature, respiration rate, heart rate, and oxygen saturation were recorded, before and after KC. Neonates were evaluated by the Neonatal Intensive Care Unit Network Neurobehavioral Scale (NNNS).
Results
Both KC groups demonstrated higher scores for attention, arousal, regulation, nonoptimal reflexes, and quality of movements and lower scores for handling, excitability, and lethargy, compared to controls (p < 0.05). Both KC groups had higher infant breastfeeding assessment tool score and reached full enteral feeds faster than controls (p < 0.05). After the first KC session, improvement in O2 saturation and temperature was observed in KC 120-min group compared with the KC 60-min group (p < 0.05). Salivary cortisol decreased in both KC groups compared with controls after 7 days (p < 0.05).
Conclusion
Preterm neonates who receive KC for long durations reach full enteral feeds faster, have better breastfeeding success, neurobehavioral performance, thermal control, and tissue oxygenation.
Similar content being viewed by others
Introduction
Prematurity is a major cause of neonatal morbidities and mortality.1 Also, premature infants are frequently exposed to many stressful and painful interventions that might lead to alterations in their stress response.2 Developmental care interventions have emerged to help premature infants to adapt to their external environment and to enhance their nervous system development.3
The World Health Organization has defined kangaroo mother care (KMC) as early, continuous, and prolonged skin-to-skin contact between a mother and her newborn with father/substitute(s) participating as KC providers; frequent and exclusive breastfeeding; and early discharge from hospital.4 However, intermittent kangaroo care (KC), for short periods once or a few times per day, for a variable number of days is commonly employed in neonatal intensive care units.5
Kangaroo care (KC) provides tactile stimulation to the infants by allowing early skin-to-skin contact between the mothers and their infants, kinesthetic-proprioceptive stimulation by direct skin-to-skin contact, olfactory-gustatory stimulation by breastfeeding, oro-motor stimulation by sucking the nipple. Further, it improves infant−mother interaction, bonding, and attachment essential for emotional and social development.6,7,8 However, the optimal duration of KC is not determined yet.
The Neonatal Intensive Care Unit Network Neurobehavioral Scale (NNNS) is a standardized assessment for the neurobehavioral integrity of the newborn;9 it examines the neurobehavioral organization, neurologic reflexes, motor development, and active and passive tone as well as signs of stress.10
This study aimed to assess the effect of KC and its duration on the neonatal neurobehavioral performance, salivary cortisol, and breastfeeding success in preterm infants. A secondary aim is to confirm the effect of KC on physiological parameters like body temperature, heart rate, respiratory rate, and oxygen saturation.
Patients and methods
This prospective double-blinded randomized controlled clinical trial was conducted on 120 stable preterm neonates with gestational age between 31 and 35 weeks at the Neonatal Intensive Care Units (NICU) of Ain Shams University Hospitals. Neonates with major congenital anomalies, perinatal asphyxia, intraventricular hemorrhage (IVH) were excluded. Also, neonates whose mothers had a history of drug abuse during pregnancy were excluded from the study.
The mothers of the newborns provided written consent for the protocol approved by the ethical committee of Ain Shams University Hospitals. This trial was registered in ClinicalTrials.gov; identifier: NCT03740594. Reporting of the study conforms to Consolidated Standards of Reporting Trials (CONSORT) 2010 statement.11
Randomization and blinding
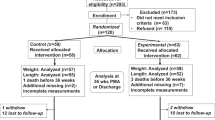
A total of 162 preterm neonates were assessed for eligibility. Initial perinatal medical severity score, called the Critical Risk Index for Babies (CRIB) Score, was recorded and only stable neonates were included.12 Forty-two neonates were excluded: 27 had hemodynamic instability (15 needed invasive ventilation, 9 needed inotropic support and 3 had hemodynamically significant patent ductus arteriosus); 5 developed IVH,1 Edwards Syndrome and 1 Down Syndrome and 8 mothers declined to participate (Fig. 1; online only).
The included 120 neonates were enrolled on the first day of life and were randomized into three groups; 40 received KC for 60 min daily for at least 7 consecutive days (KC 60-min group), 40 received KC for 120 min daily for at least 7 consecutive days (KC 120-min group), and 40 control group who received conventional neonatal care and parents were allowed to hold their babies for 15–30 min. Randomization was done using a computer-based randomization program. The allocation was concealed in sealed envelopes. During the first 24 h of life, the included neonates started the study protocol according to their group allocation. Blinding included the data collectors, NNNS examiner, outcome adjudicators, and laboratory personnel.
Clinical examination and routine neonatal care
All the studied neonates were subjected to detailed perinatal and family history, thorough clinical examination laying stress on anthropometric measurements (weight (gm), length (cm), and occipitofrontal circumference (OFC, cm)). The gestational age was determined by maternal last menstrual period and further confirmed by using the Ballard scoring system.13
The mean vital data including temperature, respiration rate (RR/min), heart rate (HR/min), blood pressure, and oxygen saturation (SpO2) were recorded, immediately before and after KC. All neonates received the standard neonatal care including adequate nutritional intake (calories, protein, calcium, phosphate) according to our NICU protocols and were followed-up clinically with at least two complete physical examinations daily (one in the morning and the other in the evening).
Neonates were assessed for the successful breastfeeding at discharge by using Infant Breastfeeding Assessment Tool (IBFAT)14 and time to reach full enteral feeding was recorded. Full enteral feeding was defined as enteral tolerance of at least 150 ml/kg/day of milk.15
Transcranial ultrasound was performed on postnatal days 1, 3 and 7 to exclude intraventricular hemorrhage. It was performed in using the two-dimensional/pulsed and color Doppler Ultrasound (LOGIQ 400 proseries General electric, Ultramark 9 Color Doppler System, Advanced Technology Laboratories, Bothell, WA, USA) with a 5 MHz transducer.
Kangaroo care technique
During the KC session, infants (wearing only a diaper) were placed upright, in a prone position, with their legs and arms flexed, and in direct skin-to-skin contact with a parent, typically their mothers’ bare chest. The mothers were reclined in a chair with a blanket draped over their chests or they could stand upright if sling or wrap was available.
The duration of the KC session was either continuous 60 min daily for at least 7 days or continuous 120 min daily for at least 7 days. All mothers in both groups were given a lecture by a trained certified nurse about KC technique and warning signs such as apnea and cyanosis. Parents were allowed to continue KC till discharge with the mean duration of KC was 12.35 ± 1.87 days in the KC 60-min group and 11.90 ± 1.37 days in the KC 120-min group (p = 0.18).
Control neonates received routine neonatal care according to our NICU protocol neonates. Parents could visit their babies anytime from 11:00 a.m. to 8:00 p.m. which are modified at certain times according to our NICU visiting hours and policies, e.g. during admission, resuscitation, procedures, interdisciplinary rounds, and nurse change of shift report. Mothers could breastfeed their babies anytime they wanted if they were stable. However, nurses wrapped the babies with blankets during breastfeeding for fear of hypothermia i.e they were not in direct skin-to-skin contact with their mothers. Otherwise, mothers bring expressed breast milk to be given to their babies by cups for the rest of the day.
Neurobehavioral assessment
The neurodevelopmental assessment was performed using the NNNS at 37 weeks gestation. The NNNS was performed 2 h after feeding. It started while the infants were in a sleep state. They then became awakened during the exam, which is reported to be the optimal state for conducting the exam.9 The neonates were examined at least 24 h away from any stressful procedures. The examination was performed by a qualified researcher with specific training in performing it. He was blinded to group distribution. The NNNS is formed of 128 items which then were summarized into 13 scores: habituation, attention, arousal, regulation, handling, quality of movement, excitability, lethargy, non-optimal reflexes, hypotonia, hypertonicity, and abstinence.16
Salivary cortisol measurement
Salivary cortisol samples were collected from all included neonates at 9 a.m. on the first day of enrollment. First KC session started in both KC groups just after salivary cortisol sampling. Another sample was taken just after finishing the first KC session in both KC groups to assess the immediate effect of KC on the cortisol level. Salivary cortisol assay was repeated in all included neonates after 7 days at 9 a.m. to assess the sustained effect of KC on serum cortisol. No painful intervention was performed at least 12 h prior to the collection of saliva.
Saliva was collected using a sterile pipette. It was placed under the tongue and close to the cheek. Salivary samples were collected at least 30 min apart from oral feeding to avoid mixing of saliva with milk. After collection, the saliva was centrifuged and stored at −70 °C. Cortisol levels were then measured using enzyme-linked immunosorbent assay (ELISA) kit supplied by DGR International, Inc, Germany. Samples were run in duplicate. Each sample was dispensed into selected well and enzyme conjugate was added, then incubated for 60 min at room temperature. Wells were then washed for three times after which substrate solution was added to each well and incubated for 30 min at room temperature. The reaction was then stopped by adding Stop Solution to each well. The absorbance of each well was determined at 450 ± 10 nm. A standard curve was constructed from which the concentrations of cortisol in the samples were determined.
Outcome measures
The following primary outcome data were recorded before the beginning of the study and after completion of the KC protocol for at least 7 days: neurodevelopmental performance using The Neonatal Intensive Care Unit Network Neurobehavioral Scale and salivary cortisol levels. Secondary outcomes included assessment of the time taken to establish full enteral feeding, evaluation of breastfeeding success using IBFAT score and recording physiological vital parameters.
Sample size calculation
A sample of at least 20 preterm neonates per group was calculated using power and sample size calculation program version 3, α error = 0.05, power of study = 90%, mean difference of 0.6 points on scores of NNNS variables between groups with a standard deviation of 0.5.17
Statistical analysis
Data were analyzed using the Statistical Package for Social Science (IBM SPSS) version 22 (SPSS Inc., Armonk, NY, USA). Normally distributed numerical variables were presented as mean ± standard deviation (SD). Quantitative nonparametric data were described in the form of median and range. Qualitative data were described as frequency and percentage. Student-t, Mann−Whitney (U), Chi-square (X2) tests were used for intergroup comparison. Pearson’s correlation (r) was used for correlating data and Spearman’s rank correlation coefficient (rs) was used for correlating between data when one or more was skewed. Paired parametric data were compared using Paired t test, paired nonparametric data were compared nonparametrically using the Wilcoxon signed ranks test. For assessing the effect of KC on neurobehavioral responses of preterm infants, controlling for the confounders, the scores of the 13 variables of NNNS were analyzed using logistic regression analysis estimating the odds ratio (OR) and 95% confidence interval (CI). p value < 0.05 was considered significant.
Results
As shown in Table 1, there was no significant difference between the studied groups regarding maternal characteristics, neonatal demographic data, and anthropometric measurements (p > 0.05). In the view of breastfeeding success, both KC groups had significantly higher IBFAT score and a shorter time to reach full enteral feeding than controls (p < 0.001). Furthermore, KC 120-min groups had higher IBFAT score than the KC 60-min group.
The mean changes in the physiological vital parameters after the first KC session (post-KC minus pre-KC) of both groups are reported in Table 2. The rise in O2 saturation and temperature was significantly higher in KC 120-min group compared with the KC 60-min group (p < 0.05). None of the infants developed instability in any of the physiological parameters, apnea or hypothermia during the session.
Table 3 shows the scores of the 13 NNNS variables for the three groups. Newborns of KC groups had higher scores for attention, arousal, regulation, nonoptimal reflexes, and quality of movements and lower scores for excitability and lethargy and a lower need for handling compared to those of the control group (p < 0.05). Newborns of KC 120-min group had higher scores for attention, arousal, regulation, and nonoptimal reflexes and lower scores for handling, excitability, and lethargy, compared to those of the KC 60-min group (p < 0.05). After controlling for confounders, logistic regression analysis showed that KC was associated with higher scores on the attention (95% CI = 2.175–17.038, p = 0.027), and lower scores on excitability (95% CI = 0.102–0.800, p = 0.017) and lethargy (95% CI = 1.455–10.352, p = 0.007).
Table 4 shows that there was no significant difference between the studied groups in the baseline salivary cortisol level. After the first KC session, salivary cortisol decreased significantly in KC groups compared with baseline level (p < 0.05), with no significant difference between both KC groups (p > 0.05) and no significant correlation between the baseline salivary cortisol levels and the baseline heart rate, respiratory rate, O2 saturation, temperature or gestational age (p > 0.05).
On day 7 from the trial entry, Fig. 2 showed that salivary cortisol level decreased significantly in all the three groups with much more decrease in both KC groups compared with controls (p < 0.001) with no significant difference between KC 60-min and 120-min groups (p = 0.793).
Discussion
In the present study, we examined the effect of KC and its duration on vital data, NNNS score, breastfeeding success and salivary cortisol. During the Neonatal Intensive Care Unit Network Neurobehavior Scale (NNNS) exam, applied at 37 post-conceptual weeks, preterm neonates with gestational age between 31 and 35 weeks who received KC for 60 or 120 min daily for at least 7 consecutive days showed better quality of movements with greater amplitude, smoother and more harmonious, greater attention and orientation to external stimuli, fewer reflexes with asymmetrical responses, a lower need for handling, and less excitability and lethargy compared with the control group and this was more prominent in neonates who received KC for 120 min. The explanation of this is that KC provides the best model of developmental care interventions as it provides multimodel sensory stimulation and the longer the duration of multi-sensory stimulation through KC, the faster the acceleration of the process of ongoing neurological maturation.18
Similarly, Feldman and Eidelman19 documented that preterm neonates who received KC for at least 1 h daily for 14 days showed better responses to external stimuli in comparison with neonates who received conventional care. Ohgi et al.20 reported that preterm neonates who received KC had better scores for attention, orientation, alertness, and less irritability. Moreover, Reynolds et al.21 found that more holding was associated with improved quality of movements, less stress, and less excitability in a previous study. Recently, Silva et al.17 showed that KC facilitates neurobehavioral organization, with better state control, more capability of habituation and orientation to external stimuli, better quality of movements and fewer signs of stress.
In the view of the effects of different KC durations, prolonged duration (continuous and overall intermittent duration) and frequent KC intervention are reported to result in increased alertness/attention and reduction in the overall likelihood of a neurodevelopmental delay.18 In a previous study, preterm babies were divided into groups according to the daily duration of KC. They reported that anthropometric parameters improved with the increase in the duration of KC.22 Another study reported that infants who received higher total hours of skin-to-skin care were more likely to score ≥ 80 on the cognitive and communication scales of the Bayley-III at 6- and 12-month assessment.23
As regards breastfeeding success, both KC groups had significantly higher IBFAT score and a shorter time to reach full enteral feeding than controls with the highest score among those who received KC for 120 min. The most substantial evidence of benefit from KC is for breastfeeding. In this context, a previous study reported that preterm neonates who could maintain breastfeeding for 6 months had spent more time in KC per day than those who could not.24 Also, previous studies reported that mothers who had more KC had more exclusive breastfeeding.25,26,27
Upon studying the effect of KC duration on the vital signs, there was a significant improvement in O2 saturation and temperature after 120 min of KC compared with 60 min KC neonates. These results are in agreement with Almeida et al.28 who found a significantly higher temperature during KC and attributed the better thermal control to the application of this method. Almeida et al.28 and Lee and Bang29 also reported an improvement in O2 saturation after 30 min of KC. The improvement in oxygenation may be explained by the fact that the upright position of KC increases the efficiency of the diaphragm and pulmonary function30 Another explanation that the neonate remains calm and comfortable in contact with its mother, which probably decreases the consumption of oxygen.28
In the current analysis, day 7 salivary cortisol, measured as an indicator of the stress response, was significantly lowered in both KC groups compared to the conventional care group with no difference between both KC groups. These results indicate that KC has a relieving effect on the infant’s stress response that could be explained by the fact that early physical contact with the mother has an impact on the infant’s neuro-endocrinal pathways that manage stress.31 Previous studies reported that neonates who received KC for more than 60 min showed a considerable drop in their cortisol level.32,33,34 The limitation of the current study is the unattainability of long-term monitoring of growth, neurodevelopment outcomes over a longer period of follow-up.
In conclusion, the present study documents that applying KC for a longer duration to stable preterm infants improves neurobehavioral performance at 37 weeks gestation, raises oxygen saturation and body temperature, shortens time to full enteral feeds, and enhances breastfeeding success, and therefore adds an important perspective to the already robust literature on this subject. Further studies with longer durations of KC and repeated exposure to KC multiple times per day are required. In addition, the optimal KC duration for unstable infants should be the target of future studies with longer follow-up to determine the impact of KC on the infant’s long-term neurodevelopmental performance.
References
Blencowe, H. et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr. Res. 74(Suppl 1), 17–34 (2013).
Vinall, J. & Grunau, R. E. Impact of repeated procedural pain-related stress in infants born very preterm. Pediatr. Res. 75, 584–587 (2014).
Ferber, S. G. & Makhoul, I. R. The effect of skin-to-skin contact (kangaroo care) shortly after birth on the neurobehavioral responses of the term newborn: a randomized, controlled trial. Pediatrics 113, 858–865 (2004).
Chan, G. J., Valsangkar, B., Kajeepeta, S., Boundy, E. O. & Wall, S. What is kangaroo mother care? Systematic review of the literature. J. Glob. Health 6, 010701 (2016).
Nyqvist, K. H. et al. Towards universal Kangaroo Mother Care: recommendations and report from the First European conference and Seventh International Workshop on Kangaroo Mother Care. Acta Paediatr. 99, 820–826 (2010).
Moore, E. R., Anderson, G. C., Bergman, N. & Dowswell, T. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst. Rev. 11, CD003519 (2012).
Ramachandran, S. & Dutta, S. Early developmental care interventions of preterm very low birth weight infants. Indian Pediatr. 50, 765–770 (2013).
Cong, X., Ludington-Hoe, S. M. & Walsh, S. Randomized crossover trial of kangaroo care to reduce biobehavioral pain responses in preterm infants: a pilot study. Biol. Res. Nurs. 13, 204–216 (2011).
El-Dib, M., Massaro, A. N., Glass, P. & Aly, H. Neurobehavioral assessment as a predictor of neurodevelopmental outcome in preterm infants. J. Perinatol. 32, 299–303 (2012).
Lester, B. M., Tronick, E. Z. & Brazelton, T. B. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics 113, 641–667 (2004).
Schulz, K. F., Altman, D. G., Moher, D. & Grp, C. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 152, 726–W293 (2010).
The International Neonatal Network. The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. The International Neonatal Network. Lancet 342, 193−198 (1993).
Ballard, J. L. et al. New ballard score, expanded to include extremely premature-infants. J. Pediatrics 119, 417–423 (1991).
Matthews, M. K. Developing an instrument to assess infant breastfeeding behaviour in the early neonatal period. Midwifery 4, 154–165 (1988).
Corvaglia, L. et al. Predictors of full enteral feeding achievement in very low birth weight infants. PLoS ONE 9, e92235 (2014).
Boukydis, C. F., Bigsby, R. & Lester, B. M. Clinical use of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics 113, 679–689 (2004).
Silva, M. G., Barros, M. C., Pessoa, U. M. & Guinsburg, R. Kangaroo-mother care method and neurobehavior of preterm infants. Early Hum. Dev. 95, 55–59 (2016).
Ludington-Hoe, S., Morgan, K. & Abouelfettoh, A. A clinical guideline for implementation of kangaroo care with premature infants of 30 or more weeks’ postmenstrual age. Adv. Neonatal Care 8, S3–S23 (2008).
Feldman, R. & Eidelman, A. I. Skin-to-skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev. Med. Child Neurol. 45, 274–281 (2003).
Ohgi, S. et al. Comparison of kangaroo care and standard care: behavioral organization, development, and temperament in healthy, low-birth-weight infants through 1 year. J. Perinatol. 22, 374–379 (2002).
Reynolds, L. C. et al. Parental presence and holding in the neonatal intensive care unit and associations with early neurobehavior. J. Perinatol. 33, 636–641 (2013).
Udani, R., Aloke, V. R., Kabra, N. & Nanavati, R. Impact of duration of Kangaroo Mother Care on growth in High risk preterm and low birth weight infants. J. Neonatol. 27, 1–9 (2013).
Gonya, J., Ray, W. C., Rumpf, R. W. & Brock, G. Investigating skin-to-skin care patterns with extremely preterm infants in the NICU and their effect on early cognitive and communication performance: a retrospective cohort study. BMJ Open 7, e012985 (2017).
Flacking, R., Ewald, U. & Wallin, L. Positive effect of kangaroo mother care on long-term breastfeeding in very preterm infants. J. Obstet. Gynecol. Neonatal Nurs. 40, 190–197 (2011).
Suman, R. P., Udani, R. & Nanavati, R. Kangaroo mother care for low birth weight infants: a randomized controlled trial. Indian Pediatr. 45, 17–23 (2008).
Briere, C. E., Lucas, R., McGrath, J. M., Lussier, M. & Brownell, E. Establishing breastfeeding with the late preterm infant in the NICU. J. Obstet. Gynecol. Neonatal Nurs. 44, 102–113 (2015).
Almeida, H., Venancio, S. I., Sanches, M. T. & Onuki, D. The impact of kangaroo care on exclusive breastfeeding in low birth weight newborns. J. Pediatr. (Rio J.) 86, 250–253 (2010).
Almeida, C. M., Almeida, A. F. N. & Forti, E. M. P. Effects of Kangaroo mother care on the vital signs of low-weight preterm newborns. Braz. J. Phys. Ther. 11, 1–5 (2007).
Lee, J. & Bang, K. S. The effects of kangaroo care on maternal self -esteem and premature infants’ physiological stability. Korean J. Women Health Nurs. 17, 454–462 (2011).
Parmar, V. R. et al. Experience with Kangaroo mother care in a neonatal intensive care unit (NICU) in Chandigarh, India. Indian J. Pediatr. 76, 25–28 (2009).
Feldman, R., Singer, M. & Zagoory, O. Touch attenuates infants’ physiological reactivity to stress. Dev. Sci. 13, 271–278 (2010).
Takahashi, Y., Tamakoshi, K., Matsushima, M. & Kawabe, T. Comparison of salivary cortisol, heart rate, and oxygen saturation between early skin-to-skin contact with different initiation and duration times in healthy, full-term infants. Early Hum. Dev. 87, 151–157 (2011).
Mitchell, A. J., Yates, C. C., Williams, D. K., Chang, J. Y. & Hall, R. W. Does daily kangaroo care provide sustained pain and stress relief in preterm infants? J. Neonatal Perinat. Med. 6, 45–52 (2013).
Mooncey, S., Giannakoulopoulos, X., GIover, V., AcoIet, D. & Modi, N. The effect of mother-infant skin-to-skin contact on plasma cortisol and β-endorphin concentrations in preterm newborn. Infant Behav. Dev. 20, 553–557 (1997).
Author information
Authors and Affiliations
Contributions
Each author has met the authorship requirements. R.E.-F. and D.M.S. contributed to the conception and design, acquisition of data, or analysis and interpretation of data, planned the study, carried out experiments and analyzed data. D.A.R., R.M.S., and W.E.S. performed the laboratory analysis and shared in writing the manuscript and analysis of data. A.S.F. contributed to the recruitment of pregnant women who are entering in preterm labor. D.H.S. shared in conducting the experiment and data interpretation. M.F.S. shared in data collection and statistical analyses. All authors were involved in drafting the article or revising it critically for important intellectual content and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
El-Farrash, R.A., Shinkar, D.M., Ragab, D.A. et al. Longer duration of kangaroo care improves neurobehavioral performance and feeding in preterm infants: a randomized controlled trial. Pediatr Res 87, 683–688 (2020). https://doi.org/10.1038/s41390-019-0558-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0558-6
This article is cited by
-
Predictors of time to full enteral feeding in low birth weight neonates admitted to neonatal intensive care unit: a prospective follow up study
BMC Pediatrics (2024)
-
A systematic review and multivariate meta-analysis of the physical and mental health benefits of touch interventions
Nature Human Behaviour (2024)
-
The effect of the swaddling method on stress levels in newborns administered nasal CPAP
BMC Pediatrics (2023)
-
NICU sensory experiences associated with positive outcomes: an integrative review of evidence from 2015–2020
Journal of Perinatology (2023)
-
Early-life factors associated with neurobehavioral outcomes in preterm infants during NICU hospitalization
Pediatric Research (2022)