Abstract
Background
Decreased expression of the renal aquaporin (AQP) protein family is associated with hydronephrosis in adult humans and animals. However, the expression of AQPs, especially subtypes AQP1–3, which play a core role in the urinary concentration function, in hydronephrotic human fetuses is not clear. The aim of this study is to investigate the expression of the AQP1–3 in normal and hydronephrotic human fetal kidneys.
Methods
Twenty-one normal and six hydronephrotic kidney (HK) samples were harvested from abortive fetuses. Meanwhile, seven normal adult human kidney samples were collected as positive controls. Quantitative real-time PCR, western blotting, and immunohistochemistry were used to analyze the expression of AQP1–3.
Results
Both the protein and messenger mRNA expression levels of AQP1–3 increased with gestational age in the normal fetuses, but the levels were significantly lower than those in the adult tissues and significantly higher than those in the hydronephrotic fetuses at the same gestational age.
Conclusions
The increased expression of AQP1–3 with gestational age in the fetal kidney may indicate maturation of the urinary concentrating ability. The lower expression of AQP1–3 in HKs may reflect a maturation obstacle with regard to urinary concentration in human hydronephrotic fetuses.
Similar content being viewed by others
Introduction
Congenital hydronephrosis (CH) is the most frequent cause of renal failure in children and infants.1,2 Antenatal screening can identify at least 20% clinical fetal hydronephrosis in 1 of 100 births.3,4 Urinary developmental abnormalities, such as congenital obstructive nephropathy resulting in CH, are responsible for up to 54% of chronic renal insufficiency cases, which can further progress into renal failure or/and lead to death after birth.2,4 However, the molecular mechanisms of CH in humans are unclear. Reduced expression of renal aquaporins (AQPs) has been demonstrated to be associated with an impaired urinary concentrating capacity in CH children;5,6 thus, renal AQPs may affect the maturation and normal development of the urinary concentrating ability of a human fetus.
Thirteen subtypes of APQs exist in mammals, and eight subtypes (AQP1, 2, 3, 4, 6, 7, 8, and 11) are expressed in the kidney,7,8,9 but AQP1, AQP2, and AQP3 are the main renal AQPs, which are essential for urinary concentration and serve as important water channels.10,11,12,13,14,15,16,17,18,19 During gestation, a large volume of hypotonic urine produced by the fetal kidney is the main source of amniotic fluid, which is essential for normal fetal development.20 Therefore, abnormal expression of these main renal AQPs may result in urinary concentrating disability, leading to aberrant amniotic fluid (oligo- and polyhydramnios). However, the literature on the expression of AQP1–3 in normal and hydronephrotic human kidneys is very limited, especially regarding the gestational period.21
Thus, this study aims to investigate the expression levels of AQP1, AQP2, and AQP3 in normal and hydronephrotic human fetal kidneys, which may provide insight into normal development of the fetal renal concentrating ability and also helps explain the molecular pathogenesis of CH.
Methods
Human tissue samples
Surgical specimens were harvested under sterile conditions from a total of 21 normal fetal kidneys (ten left and eleven right kidneys), six hydronephrotic kidneys (HKs) (two left and four right kidneys), and seven normal male adult kidneys. Seventeen cases contributed to the kidneys, eleven cases (one case with solitary kidney) contributed to the normal kidneys, six cases contributed to the HK kidneys. The kidneys were procured at The First Affiliated Hospital of Zhengzhou University. The indication for abortion was complex congenital heart disease in the fetuses. The complex congenital heart diseases included Tetralogy of Fallot (TOF), complete transposition of the great arteries (c-TGA) combined with ventricular septal defect, or c-TGA combined with ventricular septal defect and pulmonary stenosis (PS). Before abortion, all fetuses were evaluated by ultrasound and showed a normal kidney or a HK. The abortions were performed medically (ethacridine). All the fetuses had intrauterine demise, and the interval between fetal demise and tissue harvest was <1 h.
According to the Society for Fetal Urology (SFU) guidelines for grading hydronephros,22 hydronephrosis on prenatal ultrasound was defined as follows: grade 0, no HN; grade I, only the renal pelvis is visible; grade II, a few but not all calices are identifiable in addition to the renal pelvis; grade III, virtually all calices are visible; and grade IV, the calices may have a similar appearance to grade III calices, but compared with the normal side, the affected kidney exhibits parenchymal thinning.
All the HKys included in this study were SFU grade IV and unilateral. Compared with the contralateral normal kidney, the hydronephrotic fetal kidneys were larger, the renal medulla was reduced, and the renal pelvis was filled with urine. These changes were obvious and could be easily recognized by the naked eye; thus, the kidney morphology was evaluated again immediately after abortion (a representative picture of a HK (Fig. 1) is shown in the Supplemental materials).
Hematoxylin and eosin (HE) staining of the renal cortex from human fetal kidneys: Representative images of HE staining in the fetal human kidney (a, b) at 15 weeks of gestation, (c, d) at 24 weeks of gestation, (e, f) at 30 weeks of gestation, and (g, h) at 36 weeks of gestation, as well as in (i, j) an adult human kidney. Original magnification: ×50 for a, c, e, g, and i; ×400 for b, d, f, h, and j. Scale bar: 500μm for a, c, e, g, and i; 50μm for b, d, f, h, and j
For the HKs, three kidneys were harvested at 32 weeks of gestation, two kidneys were harvested at 33 weeks of gestation, and one kidney was harvested at 34 weeks of gestation. The adult kidney specimens were procured during nephrectomy for renal pelvic carcinoma, and the renal specimens were obtained from the normal part of the kidney.
To exclude differences caused by locations, all fetal specimens were collected as whole kidneys, and each specimen was divided into two parts by cutting in the sagittal plane: one part was frozen in liquid nitrogen for protein and messenger RNA (mRNA) detection, and the other part was fixed in paraformaldehyde for immunohistochemistry. When performing the experiments, a piece of a kidney specimen containing equal parts of the cortex and medulla was used.
Pregnant women who attended the hospital for an abortion or abortive induction and were suitable for sample collection were invited to join this program. We explained the research protocol to them and their families, and all parents signed the informed consent form before the abortion.
Experimental protocol
Normal fetal kidneys of different gestational ages were included to investigate the expression of AQP1–3 (Table 1), and hydronephrotic fetal kidneys of late gestational ages were included to investigate the influence of CH on the expression of AQP1–3 (Table 2).
The expression of AQP1–3 during development: The fetal kidneys were divided into four groups according to gestational age: G15–21 (n = 8, 15–21 weeks of gestation), G23–28 (n = 7, 23–28 weeks of gestation), and G30–36 (n = 6, 30–36 weeks of gestation). The adult group (n = 7) was used as a positive control.
The influence of hydronephrosis on the expression of AQP1–3 during development: The kidney specimens were divided into three groups: the normal group (n = 6, 32–34 weeks of gestation), the hydronephrotic group (n = 6, 32–34 weeks of gestation), and the adult group (n = 7), which was used as a positive control.
Immunohistochemistry
Kidneys samples were fixed with 4% paraformaldehyde buffer and washed 3 times for 10 min with phosphate-buffered saline (PBS), and then dehydrated and embedded in wax. The paraffin-embedded tissues were cut in 4-μm sections on a rotary microtome (Leica, RM2016). The sections were dewaxed and rehydrated. For immunoperoxidase labeling, endogenous peroxidases were blocked by 0.5% H2O2 in absolute methanol for 10 min at room temperature. To reveal antigens, the sections were incubated in 1 mmol/l Tris solution (pH 9.0) supplemented with 0.5 mM ethylenediaminetetraacetic acid and heated in a microwave oven for 10 min. Nonspecific binding of immunoglobulin G (IgG) was prevented by incubating the sections in 50 mM NH4Cl for 30 min, followed by blocking in PBS supplemented with 2% bovine serum albumin (BSA). The sections were incubated overnight at 4 °C with the primary antibodies rabbit anti-AQP1 (GB11310-1, Servicebio, China), anti-AQP2 (PB0499, Boster Biological Technology Co. Ltd., USA), and anti-AQP3 (BA1559, Boster Biological Technology Co. Ltd.) diluted in PBS supplemented with 0.3% BSA and 0.3% Triton X-100. The sections were rinsed with PBS 3 times for 5 min each, and then the sections were incubated with horseradish peroxidase-conjugated secondary antibodies (G23303, goat anti-rabbit Ig, diluted 1:200, Servicebio, China) for 1 h at room temperature. After rinsing with PBS washing buffer, the sites of antibody–antigen reactions were visualized with 0.05% 3,3′-diaminobenzidine tetrachloride dissolved in distilled water with 0.1% H2O2. Light microscopy was carried out with a Leica microscope. The sections taken from the adult kidneys were labeled at the same time with the same solutions to allow comparison. In addition, the kidney samples were routinely fixed, embedded in paraffin, and cut into 4-μm sections as described above. The slides were dewaxed in xylene, stained with hematoxylin and eosin, and evaluated with a Leica light microscope. The sections taken from adult normal kidney and the fetal kidney tissue were labeled at the same time with the same solutions as positive controls. Negative controls for immune-specificity were included in all experiments with PBS, using matching concentrations of normal rabbit serum. The immunohistochemistry grade of AQP expression was performed by evaluating the labeling index of renal tubules in 15 consecutive high-power (×400) fields of each section with a computer-assisted image analyzer system (ImageJ).
Quantitative real-time PCR
Total RNA was isolated and purified with TRIzol reagent (Invitrogen, USA). RNA was quantitated using a spectrophotometer (ND-1000 V3.5.2 Software, USA) and stored at −80 °C. Complementary DNA (cDNA) was synthesized using a High-capacity cDNA Reverse Transcription Kit (CW BIOTECH, China). Forty microliters of the RT reaction volume consisted of 8 µL of dNTP mix, 4 µL of primer mix, 8 µL of 5× RT buffer, 2 µL of Super RT, 12 µL of RNase-free water, and 6 µL of RNA sample (6 µg of total RNA). Reverse transcription was performed in a water bath at 42 °C for 40 min and 85 °C for 5 min. Then, the synthetic cDNAs were stored at −80 °C.
Quantitative real-time PCR (QPCR) was performed with the Applied Biosystems 7500 Sequence Detection System. The primer sequences were used according to Table 3. Twenty-five microliters of the real-time PCR reaction mixture included 1 µL of cDNA, 12.5 µL of SYBR mixture (CW BIOTECH, China), 1 µL of forward and reverse primers, and 10.5 µL of RNase-free water. Samples were amplified in duplicate 96-well plates, and PCR was performed for 40 cycles consisting of denaturation for 15 s at 95 °C, followed by annealing and polymerization at 60 °C for 1 min and a further melting-curve step at 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s and 60 °C for 15 s. Glyceraldehydes-3-phophate dehydrogenase expression was assayed for normalization. All samples used for comparison were amplified in the same “run.” The relative expression levels were determined with the change in threshold cycle (ΔCT) method, and the fold changes in gene expression were calculated by the equation 2−ΔΔCT.23
Electrophoresis and immunoblotting
Total protein was prepared from tissue samples. The kidney tissues (20 mg) were lysed in 200 µL of Tissue Protein Extraction Reagent (CW BIOTECH, China) and homogenized. The lysate was then centrifuged at 12,000 rpm for 20 min at 4 °C to remove tissue debris. The total protein concentration was determined with a Pierce BCA Protein Assay Kit (Trans, Beijing, China). For each sample, the protein extract (50 µg) was loaded and separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a 12% polyacrylamide resolving gel and a 5% polyacrylamide stacking gel. The proteins were electrophoretically transferred to a PVDF membrane. The membranes were blocked with 5% nonfat milk in Tris-buffered saline (pH 7.4) containing 0.1% Tween-20 (TBST). The membranes were then incubated overnight at 4 °C with affinity-purified, anti-rabbit polyclonal antibodies against AQP1 (Abcam, ab15080, UK), AQP2 (Abcam, ab15081, UK), AQP3 (Abcam, ab12519, UK), and β-actin (Servicebio, Gb1001, China) in TBST with 5% nonfat milk. A secondary antibody concentration of 1/7000 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used to incubate the membrane for 1 h at room temperature, and then the membranes were washed in TBST. The labeling was enhanced with an enhanced chemiluminescence (ECL) System (Thermo Fisher Scientific, USA). The labeling density was quantitated from the blots.
The experimental protocols were approved by the institutional review board and the ethics committee of The First Affiliated Hospital of Zhengzhou University (number: 2013-LW-1202).
Statistical analysis
For densitometry of immunoblots, samples from fetal kidneys were run on each gel with corresponding adult kidneys. Kidney expression of AQP1–3 in the samples from fetal kidneys was calculated as a fraction of the mean adult control value for that gel. Statistical analyses were performed with SPSS version 21 (SPSS Inc., IBM Company) and GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA). All values are presented as the interquartile range. The levels of AQP1–3 were compared among the groups using Kruskal–Willis tests, and the levels of AQP1–3 between two groups were compared using Bonferroni tests. P < 0.05 was considered significant.
Results
Morphology of fetal human kidneys during development
As shown in Fig. 1, the nephrogenic zone appeared smaller in the renal cortex from 15 to 36 weeks of gestation. In the kidney from 36 weeks of gestation, the cortex contained less interstitial tissue due to the proliferation of glomeruli and tubules during development. The glomeruli at 15 weeks of gestation were larger than those at 24, 30, or 36 weeks, but smaller than those of the adult kidneys (Fig. 1). At 15 weeks of gestation, the medulla was rich in interstitial tissue (Fig. 1a, b). From 15 to 36 weeks of gestation, the medulla had more renal tubules and capillary networks as nephrons developed (Fig. 1).
Renal mRNA expression of AQP1–3 during kidney development in the human fetus
QPCR revealed that renal AQP1–3 mRNA transcripts were detectable at 15 weeks of gestation, and the expression levels among the G15–21, G23–28, and G30–36 groups were significantly different, P < 0.05 (Fig. 2a–c), indicating that the AQP1–3 mRNA expression levels increased with gestational age, but were substantially lower than those in the adult group (Fig. 2a–c). Compared with the normal fetal kidney group, the hydronephrotic group had lower expression levels of AQP1–3 mRNA at the same gestational age (Fig. 3a–c).
The messenger RNA (mRNA) expression of AQP1–3 in fetal and adult human kidneys: Stem-and-leaf plots showing renal a AQP1, b AQP2, and c AQP3 expression in human fetuses from 15 to 36 weeks of gestation. #<0.05 compared with the adult group; *<0.05 compared with the G15–21 group; §<0.05 compared with the G23–28 group
The messenger RNA (mRNA) expression of AQP1–3 in normal and hydronephrotic fetal and normal adult human kidneys: Stem-and-leaf plots showing renal a AQP1, b AQP2, and c AQP3 expression in normal and hydronephrotic fetal kidneys and in normal adult human kidneys. #<0.05 compared with the adult group; *<0.05 compared with the normal fetal group. All the fetal kidneys are at 32–34 weeks of gestation
The location of renal AQP1–3 during kidney development in the human fetus
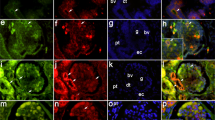
Immunohistochemical staining showed weak labeling of AQP1 in a few proximal tubules at 15 weeks of gestation (Fig. 4a). At 24, 30, and 36 weeks, AQP1 staining appeared to increase in intensity gradually over time, but was still weaker than that of the adult kidney (Fig. 4a). The labeling intensity of the HK samples was weaker than that of a normal fetal kidney samples at the same gestational age (Fig. 5a).
a Representative immunohistochemical staining images for AQP1–3 in human fetal kidneys at 15, 24, 30, and 36 weeks of gestation, as well as in an adult human kidney: original magnification: ×400. Scale bar: 50 μm. b, c Semiquantitative immunoblotting of AQP1–3 in fetal and adult human kidneys: b Immunoblots were reacted with anti-AQP1, anti-AQP2, anti-AQP3, and anti-β-actin. c Densitometric analysis revealed that the expression of AQP1, AQP2, and AQP3 increased with gestational age. #<0.05 compared with the adult group; *<0.05 compared with the G15–21 group; §<0.05 compared with the G23–28 group
a Representative immunohistochemistry staining images for AQP1–3 of normal fetal kidneys, hydronephrotic kidneys, and normal adult human kidneys: all the fetal kidneys are at 32 weeks of gestation. Original magnification: ×400. Scale bar: 50 μm. b, c Semiquantitative immunoblotting of AQP1–3 in normal and hydronephrotic fetal and normal adult human kidneys: b immunoblots were reacted with anti-AQP1, anti-AQP2, anti-AQP3, and anti-β-actin. c Densitometric analysis revealed that the hydronephrotic fetal kidneys have lower AQP1–3 expression levels. #<0.05 compared with the adult group; *<0.05 compared with the normal fetal kidneys. All fetal kidney samples are at the gestational age of 32–34 weeks
For AQP2, the immunohistochemical staining was only detectable in a few cells in the collecting ducts at 15 weeks (Fig. 4a). At 24 and 30 weeks (Fig. 4a), AQP2 labeling appeared in more collecting ducts; the staining intensity was stronger than that at 15 weeks, and even stronger staining was observed at 36 weeks of gestation (Fig. 4a), although the staining was weaker than that of the human adult kidneys (Fig. 4a). The labeling intensity of AQP2 in the HKs was weaker than that in the normal kidneys (Fig. 5a).
For AQP3, at 15 weeks, the immunohistochemical staining was weak in the basolateral collecting ducts (Fig. 4a). At 24, 30, and 36 weeks, the staining of AQP3 appeared to increase in intensity gradually over time, but was still weaker than that of the adult kidneys (Fig. 4a). The labeling intensity of the HKs was weaker than that of the normal kidneys at the same gestational age (Fig. 5a).
Thus, the immunohistochemical staining of AQP1–3 has almost the same developmental pattern, where the intensity became stronger and the amount increased with increasing gestational age.
The expression of AQP1–3 protein during kidney development in the human fetus
Immunoblotting revealed that renal AQP1–3 protein levels were detectable at 15 weeks gestation, and the expression levels increased significantly from 15 to 36 weeks of gestation (Fig. 4b, c). However, the expression levels of AQP1–3 proteins were still substantially lower than those of the adult human kidneys (Fig. 4b, c). Hydronephrotic fetal kidneys had lower expression levels of AQP1–3 at the same gestational age (Fig. 5b, c).
Discussion
The present study found that AQP1–3 proteins and mRNA were detectable in human fetal kidneys. The expression levels of renal AQP1–3 increased gradually with gestational age in normal fetuses. In addition, we found that the expression levels of renal AQP1–3 in hydronephrotic fetuses were lower than those in normal fetuses of the same gestational age.
Our study found that renal AQP1–3 were all detectable before mid-gestation (15/40 weeks) in humans, which is earlier than the detection times reported in most studied animals.24,25,26,27,28,29 Consistent with previous studies in animals,26 we found that the expression of AQP1–3 increased with gestation time during normal renal development, and the glomeruli were larger and interstitial tissue was richer at 15–20 weeks of gestation, with more glomeruli and tubules appearing at the late (30–36 weeks) gestation stage.
CH is a common malformation in the urinary tract system and can be easily detected by ultrasound examination during pregnancy. However, the molecular pathogenesis remains unclear.18,19,30 CH is also associated with decreased expression of the main renal AQPs;31,32,33 thus, AQP1–3 may play significant roles in impaired water management by the kidneys.
AQP1–3 has been found to play important roles in the intact urinary concentrating capacity of the kidneys. AQP1- or AQP3-knockout mice exhibit severe polyuria and an impaired urinary concentrating capacity, and children with hydronephrosis show reduced renal AQP2 expression.5,10,11,18 The above evidence suggests that decreased AQP1, AQP2, or AQP3 level may be responsible for the impaired urinary concentrating capacity. In this study, we found that the expression levels of AQP1–3 protein and mRNA were decreased compared with those in normal fetal kidneys, which is consistent with previous studies.18 The underlying mechanisms responsible for the downregulation of these AQPs require further exploration. Studies have demonstrated that urinary obstruction-induced hydronephrosis is associated with inner medullary cyclooxygenase-2 (COX-2) production, which is paralleled by downregulation of AQP2 and can be prevented by COX-2 inhibition.34 The data suggest that downregulation of these transporters may be associated with inflammatory mediators. However, other local yet unknown effects or system changes may also be responsible for the decreased expression of these transporter proteins.
Limitations of this study
Because collecting urine and blood from human fetuses is impossible, the corresponding renal function was not detected. However, during human development from the mid to late stage of gestation, fetal urine is the main source of amniotic fluid,35 which may serve as a potential specimen that can reflect the physiology of fetal urine. Thus, more studies are needed to investigate the roles of AQPs related to renal functions during human development. We did not measure the amount of amniotic fluid surrounding these fetuses, but we will conduct such measurements in future research. In addition, the fetuses with hydronephrosis may have had polyuria, but pregnancies with fetuses exhibiting hydronephrosis do not show polyhydramnios. The reason for this observation is still unclear, and further study is necessary in the future.
Since obtaining an absolute normal adult human kidney specimen is impossible, we were required to obtain positive control samples from renal pelvic carcinoma patients’ kidneys, which had relatively normal kidney tissues far from the lesions; however, some differences in APQ expression may exist between normal adult renal tissue and renal carcinoma tissue, but the expression levels of AQP1–3 were still high in our adult samples. Ethacridine was the abortion-inducing agent used in the study, even though there was no research about the influence of ethacridine on the fetal renal expression of AQPs, but it may have. In the future, we will keep an eye on this issue.
In summary, the expression of AQP1–3 increased with gestational age in the fetal kidney, which may indicate maturation of the urinary concentrating ability. The lower expression levels of AQP1–3 in HKs may reflect a maturation obstacle to urinary concentration during kidney development in human hydronephrotic fetuses; however, the specific influences require further investigation.
References
Shimada, K. et al. Urological emergency in neonates with congential hydronephrosis. Int. J. Urol. 14, 388–392 (2007).
Chevalier, R. L. Molecular and cellular pathophysiology of obstructive nephropathy. Pediatr. Nephrol. 13, 612–619 (1999).
McDill, BradleyW. et al. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc. Natl. Acad. Sci. USA 103, 6952–6957 (2006).
Ardissino, G. et al. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics 111, e382–e387 (2003).
Wen, J. G. et al. Expression of renal aquaporins is down-regulated in children with congenital hydronephrosis. Scand. J. Urol. Nephrol. 43, 486–493 (2009).
Li, Z. Z. et al. Decrease of renal aquaporins 1–4 is associated with renal function impairment in pediatric congenital hydronephrosis. World J. Pediatr. 8, 335–341 (2012).
Parreira, K. S. et al. Expression patterns of the aquaporin gene family during renal development: influence of genetic variability. Pflug. Arch. 458, 745–759 (2009).
Zelenina, M., Zelenin, S. & Aperia, A. Water channels (aquaporins) and their role for postnatal adaptation. Pediatr. Res. 57, 47R–53R (2005).
Nielsen, S. et al. Aquaporins kidney: from molecules to medicine. Mol. Med. Physiol. Rev. 82, 205–244 (2002).
Schnermann, J. et al. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc. Natl. Acad. Sci. USA 95, 9660–9664 (1998).
Chou, C. L. et al. Reduced water permeability and altered ultrastructure in thin descending limb of Henle in aquaporin-1 null mice. J. Clin. Invest. 103, 491–496 (1999).
Ma, T. et al. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J. Biol. Chem. 273, 4296–4299 (1998).
Kwon, T. H. et al. Aquaporins in the kidney. Handb. Exp. Pharmacol. 190, 95–132 (2009).
Liu, H. et al. Aquaporin gene expression and regulation in the ovine fetal lung. J. Physiol. 551, 503–514 (2003).
Robben, J. H. & Knoers, N. V. Deen PM.Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am. J. Physiol. Ren. Physiol. 291, F257–F270 (2006).
Boccalandro, C. et al. Characterization of an aquaporin-2 water channel gene mutation causing partial nephrogenic diabetes insipidus in a Mexican family: evidence of increased frequency of the mutation in the town of origin. J. Am. Soc. Nephrol. 15, 1223–1231 (2004).
Frigeri, A. et al. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc. Natl. Acad. Sci. USA 92, 4328–4331 (1995).
Ecelbarger, C. A. et al. Aquaporin-3 water channel localization and regulation in rat kidney. Am. J. Physiol. 269, F663–F672 (1995).
Ma, T. et al. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc. Natl. Acad. Sci. USA 97, 4386–4391 (2000).
Lindower, J. B. Water balance in the fetus and neonate. Semin. Fetal Neonatal Med. 22, 71–75 (2017).
Xing, L. et al. Ontogeny of the mammalian kidney: expression of aquaporins 1, 2, 3, and 4. World J. Pediatr. 10, 306–312 (2014).
Nguyen, H. T. et al. The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J. Pediatr. Urol. 6, 212–231 (2010).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Yamamoto, T. et al. Expression of AQP family in rat kidneys during development and maturation. Am. J. Physiol. 272, F198–F204 (1997).
Baum, M. A. et al. The perinatal expression of aquaporin-2 and aquaporin-3 in developing kidney. Pediatr. Res. 43, 783–790 (1998).
Smith, B. L. et al. Concurrent expression of erythroid and renal aquaporin CHIP and appearance of water channel activity in perinatal rats. J. Clin. Invest. 92, 2035–2041 (1993).
Wintour, E. M. et al. Ovine AQP1: cDNA cloning, ontogeny, and control of renal gene expression. Pediatr. Nephrol. 12, 545–553 (1998).
Butkus, A. et al. Ovine aquaporin-2: cDNA cloning, ontogeny and control of renal gene expression. Pedia. Nephrol. 13, 379–390 (1999).
Xing, L. & Nørregaard, R. Influence of sex on aquaporin1-4 and vasopressin V2 receptor expression in the pig kidney during development. Pediatr. Res. 80, 452–459 (2016).
Chevalier, R. L. Chronic partial ureteral obstruction and the developing kidney. Pediatr. Radiol. 38, S35–S40 (2008).
Manucha, W. Biochemical molecular markers in unilateral ureteral obstruction. Biocell 31, 1–12 (2007).
Frøkiaer, J. et al. Renal aquaporins and sodium transporters with special focus on urinary tract obstruction. APMIS Suppl. 109, 71–79 (2003).
Shi, Y. et al. Neonatal ureteral obstruction alters expressionof renal sodium transporters and aquaporin water channels. Kidney Int. 66, 203–215 (2004).
Nørregaard, R. et al. COX-2 activity transiently contributes to increased water and NaCl excretion in the polyuric phase after release of ureteral obstruction. Am. J. Physiol. Ren. Physiol. 292, F1322–F1333 (2007).
Lindower, J. B. Water balance in the fetus and neonate. Semin. Fetal Neonatal Med. 22, 71–75 (2017).
Acknowledgements
We would like to thank Kai Yang, who was the head nurse in the delivery room, and Xianlan Zhao, chief of the Department of Obstetrics, for helping us to collect the fetal kidney samples. We also would like to thank Xiaoping Shang, the statistician of the Department of Medical Record Room, for the statistical analysis help. This study was funded by the National Science Foundation of China (81370869 and 81370689).
Author information
Authors and Affiliations
Contributions
J.W., J.F., S.Y., Y.C., L.H., L.W., X.G., Y.W., Y.L., X.H., Z.H., C.R., Z.J., Z.G., R.Z., J.W., and J.W. made substantial contributions to the conception and design of the study, acquisition of the data, or analysis and interpretation of the data; J.F., J.W., S.Y., Y.C., L.H., L.W., X.G., and Y.W. drafted the article or revised it critically for important intellectual content; J.F., L.H., and J.W. revised the manuscript. J.W. provided final approval of the version to be published. J.F., S.Y., and Y.C. made equal contributions to the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures in this study were performed in accordance with the ethical standards of the institutional research committee and in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Feng, J., Yan, S., Chen, Y. et al. Aquaporin1–3 expression in normal and hydronephrotic kidneys in the human fetus. Pediatr Res 86, 595–602 (2019). https://doi.org/10.1038/s41390-019-0485-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0485-6
This article is cited by
-
Erythropoietin prevented the decreased expression of aquaporin1–3 in ureteral obstructive kidneys in juvenile rats
Pediatric Research (2023)
-
Alteration of N6-methyladenosine epitranscriptome profiles in bilateral ureteral obstruction-induced obstructive nephropathy in juvenile rats
Pediatric Research (2023)