Abstract
Background
Promoting a healthy intestinal microbiota may have positive effects on short- and long-term outcomes in very low birth weight (VLBW; BW < 1500 g) infants. Nutrient supply influences the intestinal microbiota.
Methods
Fifty VLBW infants were randomized to an intervention group receiving enhanced nutrient supply or a control group. Fecal samples from 45 infants collected between birth and discharge were analyzed using 16S ribosomal RNA (rRNA) amplicon sequencing.
Results
There was considerable individual variation in microbiota development. Microbial richness decreased towards discharge in the controls compared to the intervention group. In the intervention group, there was a greater increase in diversity among moderately/very preterm (MVP, gestational age ≥ 28 weeks) infants and a steeper decrease in relative Staphylococcus abundance in extremely preterm (EP, gestational age < 28 weeks) infants as compared to controls. Relative Bifidobacterium abundance tended to increase more in MVP controls compared to the intervention group. Abundance of pathogens was not increased in the intervention group. Higher relative Bifidobacterium abundance was associated with improved weight gain.
Conclusion
Nutrition may affect richness, diversity, and microbiota composition. There was no increase in relative abundance of pathogens among infants receiving enhanced nutrient supply. Favorable microbiota development was associated with improved weight gain.
Similar content being viewed by others
Introduction
The aim of nutritional management of preterm infants is to achieve postnatal growth similar to normal fetal growth.1 Despite increased focus on optimizing nutrient supply, postnatal growth failure is still common among preterm infants.2 Faster growth between birth and term equivalent age has been associated with improved cognitive development.3
Intestinal microbial colonization increases rapidly after birth and is affected by several factors such as delivery mode, exposure to antibiotics, infections, and nutrition.4 In vaginally delivered term-born infants facultative anaerobic Proteobacteria are gradually replaced by anaerobes such as Bifidobacterium, members of Clostridiales, and Bacteroides.5 Preterm infants have less bacterial diversity and increased amounts of potential pathogens compared to infants born at term.6 They often have a high abundance of enterobacteria, staphylococci, and enterococci, and low and delayed colonization with Bifidobacterium.7
The microbiota is important for intestinal maturation, maintenance of the mucosal surface integrity, and protection against pathogens.6 Alterations in the pattern of microbial colonization in preterm infants are associated with increased risk of necrotizing enterocolitis (NEC) and sepsis.8,9 The microbiota may affect growth, the immune system, neurodevelopment, and future risk of developing metabolic syndrome.6,10,11 Thus, promoting healthy intestinal microbiota in preterm infants may have positive effects on short- and long-term health outcomes.
Nutrition influences intestinal microbiota.12,13,14 Furthermore, the intestinal microbiota may affect metabolism, through catabolism of nutrients, production of energy, and synthesis of vitamins.6 Preterm infants receive parenteral nutrition (PN) until they tolerate full enteral feeding. Human milk is recommended, but must be fortified to meet the infants’ nutrient requirements.1 There is limited knowledge on how nutrient supply affects the microbiota in preterm infants.
In a randomized controlled trial (RCT) of very low birth weight (VLBW, birth weight (BW) < 1500 g) infants, we observed that enhanced nutrient supply improved postnatal growth.15 We also demonstrated that intestinal microbiota development among infants in this cohort was associated with postnatal age and post-menstrual age (PMA).16 Microbiota development among extremely preterm infants (EP, gestational age (GA) < 28 weeks) appeared delayed when compared to microbiota development of moderately/very preterm (MVP, GA ≥ 28 weeks) infants.16 However, when adjusting for PMA, microbiota development among EP infants was more similar to that of MVP infants. Richness increased after birth with an initial dominance of Staphylococcus, followed by Enterococcus, Enterobacter, and finally Bifidobacterium.16 The Enterococcus phase was mainly observed among EP infants and may have delayed microbiota maturation.16 We also observed that antibiotic use had a strong, but temporary effect on microbiota development and that the response depended on the sensitivity of the microbe to the antibiotic.16 In the present study, we describe the effects of nutrient supply on microbiota development in VLBW infants.
Methods
Study design
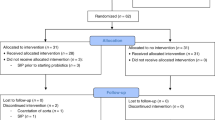
The RCT was conducted in 2010 at Akershus University Hospital and Oslo University Hospital. The study was approved by the Regional Committee for Medical and Health Research Ethics and registered in ClinicalTrials.gov (NCT01103219). VLBW infants were eligible for inclusion. Exclusion criteria were congenital malformations, chromosomal abnormalities, critical illness with short life expectancy, and clinical syndromes known to affect growth and development. A sample size of 120 infants in each group was calculated based on the primary outcome of the RCT, which was to reduce the proportion of postnatal growth-restricted infants.15 Infants were recruited by a primary investigator or the attending physician and were randomized (computer-generated block randomization) to an intervention or a control group. Sealed opaque envelopes were opened after informed parental consent was obtained. The study was not blinded. Infants were classified as either EP or MVP. After inclusion of 50 infants, a safety analysis revealed increased incidence of late-onset sepsis in the intervention group and further inclusion was stopped.15 Three infants died (control group) and two infants were excluded due to critical illness and congenital heart disease (control group), leaving 45 infants with fecal samples for analysis (Fig. 1). Forty-four infants had complete nutritional and growth data for the first four postnatal weeks (Fig. 1).
Nutritional intervention
A detailed description of the nutrition protocol has been reported previously.15 The intervention group started with 3.5 g amino acids/kg/day and 2.0 g/kg/day of a lipid emulsion containing fish oil (SMOF, Fresenius Kabi, Norway). The control group started with 2.0 g amino acids/kg/day and 0.5 g/kg/day of a lipid emulsion (ClinOleic, Baxter, Norway). Parenteral supply was gradually increased in both groups during the first days of life. All infants received minimal enteral feeding with human milk (mother’s or donor milk) in similar volumes from the first day of life (Table 1, Supplemental Fig. S1). Donor milk was frozen and not pasteurized. As enteral milk volumes were increased, parenteral supply was reduced. Time to reach full enteral feeding was similar between groups (Table 1). In both groups, fortification with Nutriprem® (Nutricia, Norway) was initiated when 110 mL/kg/day of milk was tolerated (Table 1). The intervention group received additional supply of amino acids (0.6 g Complete Amino Acid Mix®/100 mL human milk, Nutricia). From day 6, the intervention group received 60 mg/kg/day each of docosahexaenoic acid (DHA) and arachidonic acid (ARA) (Formulaic, Martek Biosciences Corporation, Columbia, MD, USA) diluted in MCT oil as well as 1500 µg/kg/day of vitamin A (Aas Laboratory, Norway) diluted in groundnut oil. The intervention group received significantly more energy, fat, protein, vitamin A, ARA, and DHA as compared to the control group, whereas enteral feeding volumes and carbohydrate supplies were similar (Supplemental Fig. S1). Median supply of energy and nutrients were higher in the intervention group as compared to the control group during the first 4 weeks: energy (139 kcal/kg/day vs. 126 kcal/kg/day, P < 0.001); fat (7.3 g/kg/day vs. 5.9 g/kg/day, P < 0.001); protein (4.0 g/kg/day vs. 3.2 g/kg/day, P < 0.001); DHA (87 mg/kg/day vs. 36 mg/kg/day, P < 0.001); ARA (68 mg/kg/day vs. 24 mg/kg/day, P < 0.001); vitamin A (1300 µg/kg/day vs. 252 µg/kg/day, P < 0.001).15 The nutritional intervention continued to 52 weeks PMA or until a body weight of 5.5 kg was reached.
Anthropometric data
Weight, length, and head circumference (HC) were registered at birth and during hospital stay. Non-sex-specific z-scores for length and HC were calculated using Fenton growth charts until 36 weeks PMA (http://uccalgary.ca/fenton). Sex-specific weight z-scores were obtained from Skjaerven’s growth charts until 36 weeks PMA.17
Fecal sampling
Fecal samples were collected approximately every 1–2 weeks from birth until discharge. Fresh samples were collected from diapers, frozen at −80 °C, and kept frozen during transport to the laboratory for analysis.
Sequencing and preprocessing
The microbial composition of fecal samples was quantified performing 16S rRNA amplicon sequencing using the Illumina MiSeq system. DNA was extracted using the repeated bead beating and the V1–V3 primers forward AGAGTTTGATCMTGGCTCAG and reverse GTATTACCGCGGCTGCTG were used for sequencing.18 The sequences were pre-processed using the R-package mare,19 applying USEARCH for quality filtering, clustering of sequences into species-like operational taxonomic units (OTUs), and for taxonomic annotation.20 We did not assign taxonomies to the OTUs because OTU clustering is a potential source of taxonomic errors. Instead, we assigned taxonomies to the sequences and formed taxonomic tables directly based on the abundance of the annotated sequences. We used Silva as a reference database, refined by excluding non-gut-associated taxa.21 Our approach was validated by analyses of artificial microbial communities of known composition.19 We only used quality-filtered forward sequences truncated to 150 nucleotides (nt) as this approach removes low-quality bases at the end of the sequence.16 The results of this approach correspond better to the actual composition in artificial communities, as compared to using long, merged paired-end sequences.15 Furthermore, we removed all sequences representing <0.001% of the total sequences because rare sequences are likely to contain errors or chimaeras. After quality filtering, the average number of sequences per sample was 68,000, ranging from 23 to 282,000. The sequencing run also contained 14 empty samples as negative controls.
Calculations of microbial indexes
Microbial richness was measured as the number of species-like OTUs. Diversity was estimated using the inverse Simpson diversity index. Microbiota development index was calculated as the sum of the first two principal coordinates as previously described.16 The principal coordinate’s analysis summarizes the microbiota into a few coordinates. The first two coordinates, especially the sum of their scores, strongly correlate with infant age and is therefore an indication of microbiota development.16
Statistical analyses
Group differences in clinical characteristics and anthropometric data were evaluated with Student’s t tests or Mann–Whitney’s U test for continuous variables, and χ2 or Fisher’s exact tests for categorical variables. Results are presented as means (ranges/standard deviations), frequencies (%), or medians (interquartile ranges).
Microbiota responses were modeled using a repeated-measures linear mixed model. The model was constructed with random intercepts for each infant, and random slope for each group. This approach permitted infants to have unique starting scores, and allowed responses over time to vary depending on different conditions. The mixed model tested postnatal age, group, and postnatal age by group effects and included GA at birth, total sequence numbers, and use of antibiotic within 5 days prior to fecal sampling as covariates. Adjustment for BW or birth BW z-score were also performed. The infants were regrouped into those: (1) with positive or negative changes in weight z-scores; and (2) receiving PN for <10 days or PN for ≥10 days. Subsequently, the data were reanalyzed using the same mixed models as described above, but with new grouping factors. In addition to BW or BW z-scores, these models were adjusted for other covariates that differed between the groups (small for gestational age (SGA), incidence of sepsis, and the volume of human milk intake first day of life). For the PN analyses, the first fecal samples obtained after the PN period were regarded as baseline for each infant.
If necessary, data was log-transformed (ln) to approximate normal distribution. Significance was assumed for P < 0.05. Statistical analyses were performed in SPSS, version 24 (SPSS, Chicago, IL, USA) and R version 3.5.1 (R Core Team 2014).
Results
Clinical characteristics and outcomes
Clinical characteristics, anthropometric measurements, nutrition, and antibiotic treatment are presented in Table 1. BW was significantly lower in the intervention group as compared to the control group. When the group was split into EP and MVP infants, there were no significant differences in anthropometric measures at birth between the EP intervention and EP control groups. In contrast, among the MVP infants, the intervention group had significantly lower weight, weight z-score, HC and HC z-scores at birth as compared to the controls. The intervention group showed improved weight and head growth at 36 weeks PMA compared to the controls,15 and among the MVP infants the intervention group also demonstrated better linear growth (Table 1).
Sepsis was more common among infants in the intervention group as compared to controls, albeit the difference was not significant in MVP and EP groups (Table 1). All except two infants received intravenous antibiotics, which was usually started within the first day of life (Table 1). Although mean days with antibiotics were higher in the intervention group, the difference was not significant (Table 1). The first antibiotic course was gentamycin usually combined with ampicillin. Later, gentamycin, ampicillin/ekvacillin, vancomycin, cephalosporins or metronidazole were used.
Nutrition and microbiota development
Two hundred and sixty-four fecal samples were collected from 45 infants between birth and discharge (Supplemental Table S1). There was considerable individual variation in microbiota development and composition (Fig. 2a–h). Microbial richness increased after birth (β = 0.35 OTUs per week, P < 0.001) and peaked between postnatal day 20 and 50 depending on groups (Fig. 2a). Following this peak, infants in the intervention group maintained their richness levels, whereas the control group exhibited a decline (β = −0.43 OTUs per week, P = 0.010). Microbial diversity increased with postnatal age (β = 0.08 per week, P = 0.008), peaking around postnatal day 20–55 depending on the groups (Fig. 2b). Among MVP infants, the intervention group demonstrated a greater increase in microbial diversity up until postnatal day 40 as compared to MVP controls (β = 0.23 per week, P < 0.001). In EP infants, we observed no significant differences in microbial diversity responses between the control and intervention group. After the peak, microbial diversity decreased in all groups until the end of the study period (β = −0.23 per week, P = 0.009) with no significant differences between the groups (Fig. 2b). The microbiota development index increased with postnatal age (β = 0.22 per week, P < 0.001) (Fig. 2c). We observed no significant effect of the nutritional intervention on the microbiota development index.
Associations between nutrient supply and microbiota. Differences between the intervention and control group according to postnatal age, in extremely preterm (EP) and moderately/very preterm (MVP) infants for a microbial richness; b microbial diversity; c microbiota developmental index, and the relative abundance of d Staphylococcus; e Enterococcus; f Enterobacter; g Bifidobacterium, and h the sum of low abundance microbes (Supplementary Fig. 2). Each line represents the time response for one infant, and is colored by its group participation. Best-fit lines (third polynomials) are presented for each group along with standard errors (shaded areas)
There were large individual variances in the abundances of different microbes (Fig. 2d–g and Supplementary Fig. S2). Staphylococcus abundance was high directly after birth, but decreased in MVP (β = −18.1%, P = 0.003) and EP groups (β = −7.7%, P < 0.001) to very low levels between postnatal days 20 and 40 (Fig. 2d). This decrease was similar for the control and intervention MVP groups, but steeper in EP intervention group as compared to EP control group (β = −38.5% per week, P = 0.035). However, the initial relative abundance of Staphylococcus appeared higher in EP intervention group infants as compared to EP controls (Fig. 2d). The relative abundance of Enterococcus increased to a peak around postnatal day 15 for MVP (β = 5% per week, P = 0.010) and 25 for EP infants (β = 20.4% per week, P < 0.001), and subsequently decreased to low levels between postnatal days 45 and 55 (Fig. 2e). The relative abundance of Enterobacter increased after birth to a peak around postnatal day 20 in MVP (β = 7.6% per week, P < 0.001), and postnatal day 50 in EP infants (β = 3.7% per week, P < 0.001). There were no significant effects of nutritional intervention on the relative abundance of Enterococcus and Enterobacter (Fig. 2e, f). The relative abundance of Bifidobacterium abundance increased after birth in MVP infants (β = 8.4% per week, P < 0.001) and EP infants (β = 3.0% per week, P < 0.001) (Fig. 2g). Among MVP infants, the control group exhibited a tendency to a greater increase in Bifidobacterium abundance as compared to the intervention group (3.2% per week, P = 0.056).
The sum of the remaining minor microbial communities decreased from birth until postnatal day 30 (β = −4.5% per week on average across all groups, P < 0.001) (Fig. 2g). This decrease may reflect the initial decrease in Bacillales abundance (Supplemental Fig. S2a). The relative abundance of the remaining minor microbial communities increased from postnatal day 30 among EP infants as compared to MVP infants (β = 4.6% per week, P = 0.014). Among EP infants, the intervention group exhibited a steeper increase in the abundance of the remaining minor microbial towards the end of the study, as compared to the control group (3.9% per week, P = 0.015) (Fig. 2h). The low abundance microbes are presented in Supplemental Fig. 2. Briefly, the relative abundance of Escherichia–Shigella (average abundance 2.65%) increased with age (β = 1.6% per week across all groups, P < 0.001), but less among infants in the intervention group as compared to controls (β = −0.69% per week, P < 0.001). The relative abundance of Klebsiella (average abundance 2.59%) was briefly lower in EP infants in the intervention group between days 21 and 29, as compared to the other groups (β = −13.3%, P = 0.005). The relative abundance of Propionibacterium (average abundance 0.31%) was higher in the intervention group infants as compared to controls (β = 0.28%, P = 0.002). Clostridium abundance was very low (average abundance 0.03%), and we were unable to perform statistical inference with certainty (data not shown).
Microbial responses and growth
Fourteen (61%) infants in the intervention group improved their weight z-scores, whereas nine (39%) had negative changes in weight z-scores between birth and 36 weeks PMA. This was significantly different (p = 0.002) from the control group, where 3 (14%) infants had a positive weight z-score change and 18 (86%) had a negative weight z-score change. The clinical characteristics and outcomes of the infants with positive and negative z-score changes are compared in Supplemental Table 2. The infants with improved weight z-scores changes had lower BW z-scores, birth HC z-scores, and more infants were SGA at birth as compared to infants with negative weight z-score changes. Infants with better growth also received higher volumes of human milk during the first day of life. Infants with a positive change in weight z-scores between birth and 36 weeks PMA (n = 17) exhibited a larger increase in microbial richness (β = 0.27 per week, P = 0.028) and in relative abundance of Bifidobacterium (β = 2.30% per week, P = 0.027) as compared to infants with a negative change weight z-score (n = 27) (Fig. 3). We did not detect any significant associations to low abundance microbes (Supplementary Fig. 3). Adjusting for BW z-scores and SGA status at birth did not change the conclusion of the results. The difference in richness was no longer significant after adjusting for volume of human milk supply during first day of life (P = 0.25).
Associations between weight z-scores change and microbiota. Differences between infants with positive vs. negative weight z-scores change according to postnatal age for a microbial richness; b microbial diversity; c microbiota developmental index, and the relative abundance of d Staphylococcus; e Enterococcus; f Enterobacter; g Bifidobacterium, and h the sum of low abundance microbes (Supplementary Fig. 3). Each line represents the time response for one infant, and is colored by its group participation. Best-fit lines (second polynomials) are presented for each group along with standard errors (shaded areas)
Microbial responses and PN
All infants received PN. We compared microbiota responses between infants receiving PN for <10 days (mean 7.6 days, n = 31) with infants in need of PN ≥10 days (referred to as prolonged PN, mean 16.1 days, n = 14). Clinical characteristics and outcomes among infants in the PN groups are compared in Supplemental Table 2. The infants receiving prolonged PN, had lower anthropometric measures at birth, higher incidence of sepsis, and more days on antibiotics as compared to infants on PN for <10 days. Infants with prolonged PN exhibited reduced microbial diversity (β = −0.37, P = 0.043) and reduced microbial richness (β = −1.2, P = 0.050) the first 33 days after birth (Fig. 4a, b), but no differences were observed thereafter (Fig. 4a, b). We observed no significant difference in the microbiota development index. The relative abundance of Enterococcus was higher the first 26 days (β = 24.8, P = 0.022) among the infants on prolonged PN, but no differences were observed thereafter (Fig. 4e). We observed no significant differences in abundances of Staphylococcus, Enterobacter, or Bifidobacterium (Fig. 4d, f, g). The differences were no longer significant after adjusting for BW, but infants with prolonged PN exhibited a tendency for lower microbial diversity (P = 0.073) and richness (P = 0.078). After adjusting for sepsis, there were no significant differences between PN groups. The sum of abundances of minor microbial communities was lower until 55 days after birth (β = −8.1, P = 0.044) among the infants on prolonged PN, but no difference was observed thereafter (Fig. 4h). We did not detect any significant associations to specific low abundance microbes (Supplementary Fig. 4).
Associations between prolonged parenteral nutrition and microbiota. Differences between infants receiving parenteral nutrition ≥10 days (prolonged parenteral nutrition) or parenteral nutrition < 10 days for a microbial richness; b microbial diversity; c microbiota developmental index, and the relative abundance of d Staphylococcus; e Enterococcus; f Enterobacter; g Bifidobacterium; and h the sum of low abundance microbes (Supplementary Fig. 4). Each line represents the time response for one infant, and is colored by its group participation. Best-fit lines (second polynomials) are presented for each group along with standard errors (shaded areas)
Discussion
We compared enhanced nutrient supply to standard nutrient supply among VLBW infants and investigated the effects on intestinal microbiota development. The microbial responses varied considerably between individuals and few significant differences were detected when comparing the groups with different nutrient supplies. Infants receiving increased nutrient supply appeared to maintain microbial richness levels better than infants on standard nutrient supply. Moreover, in the early samples MVP infants with increased nutrient supply had a steeper increase in diversity and EP infants with increased nutrient supply had a more pronounced decline in Staphylococcus as compared to infants with standard supply. However, the relative abundance of Bifidobacterium tended to increase more among MVP infants with standard nutrient supply as compared to MVP infants with increased nutrient supply. The intervention did not appear to affect the abundance of Enterococcus or Enterobacter or enhance the abundance of other potential pathogenic bacteria. In additional analyses, we observed that a larger increase in richness and relative abundance of Bifidobacterium was associated with improved weight z-scores.
Microbial richness and microbiota development index increased with postnatal age among the infants in our study.16 The initial increase in richness was not affected by increased nutrient supply. After richness peaked, the levels declined among the infants with standard nutrient supply as compared to infants with increased supply, who seemed to maintain their richness levels better. Furthermore, we observed a larger increase in microbial diversity among the MVP infants receiving enhanced nutrients as compared to the infants on standard supply. Previous studies have reported that preterm infants have reduced richness and diversity.7,22 Chernikova et al.22 showed that preterm infants had lower richness than term-born controls and that diversity declined with decreasing GA at birth. Low microbial diversity along with disruption of normal bacterial colonization may increase the risk of developing sepsis and NEC in preterm infants.23,24,25,26
Four major bacterial genera were detected in the fecal samples of the infants in our study; Staphylococcus, Enterococcus, Enterobacter, and Bifidobacterium.16 We observed a steeper decline in Staphylococcus in the EP intervention group compared to the EP controls. However, the relative abundance of Staphylococcus was higher just after birth in the EP intervention group and this may have affected the results, despite adjusting for baseline variation. Staphylococcus are neonate-type bacteria that quickly decline after birth.27,28
The relative abundance of Bifidobacterium increased with postnatal age, but there was a tendency to a greater increase among the MVP infants receiving standard nutrient supply as compared to the MVP infants receiving increased nutrients. However, the lower relative abundance of Bifidobacterium in the MVP infants with increased nutrients could be a consequence of higher abundance of other microbes and not necessarily less Bifidobacterium, suggested by the increase in diversity and maintenance of richness seen in this group. Other studies show that preterm infants have very low abundance of Bifidobacterium.7,22 A recent study reported Bifidobacterium abundance of 3% in preterm samples and 15% in term samples during the first 6 weeks of life.22 In contrast to other preterm cohorts, our infants had more Bifidobacterium in their later samples and they even obtained similar levels to that of term-born infants.22 All infants in our cohort received equal amounts of unpasteurized human milk from the first day of life. Human milk oligosaccharides, naturally found in mother’s milk, promote Bifidobacterium colonization.14
We did not detect significant differences between the nutrient groups in the alterations of Enterococcus. Enterococcus has been identified in fecal samples of both term and preterm infants.7,29 The implication of Enterococcus colonization in preterm infants’ intestines is not clear. Whereas Enterococcus may be a potential pathogen,30 this microbe has been found to modulate inflammation31 and to be more frequent among healthy preterm infants as compared to those with NEC or sepsis.32 However, we previously observed that increased relative abundance of Enterococcus may inhibit normal microbiota succession.16
Dysbiosis is a distortion in normal development of the intestinal microbiota, and may increase the risk of NEC and sepsis in preterm infants.24,26 Although different microbes have been seen in association with disease, increased abundance of gammaproteobacteria and less Bifidobacterium have been seen in infants with NEC.10,23,24,33 We did not observe any effects of increased nutrient supply on the relative abundance of Enterobacter. In general, we found very low abundances of other potential pathogens such as Klebsiella, Escherichia–Shigella, and Clostridum.30,33 We speculate that the paucity of these microbes, similar to the higher relative abundance of Bifidobacterium, may be due to the beneficial effects of early use of human milk. Enhanced nutrient supply did not increase the abundance of potential pathogens. On the contrary, we observed a brief period of lower abundance of Klebsiella among EP infants receiving increased amounts of nutrients, as compared to the other study groups. The relative abundance Escherichia–Shigella increased more among infants with standard nutrient supply as compared to infants with enhanced nutrient supply. A recent study observed that Escherichia–Shigella abundance increased prior to late-onset NEC, whereas Clostridium abundance increased before early-onset NEC.33
The intervention group had a higher relative abundance of Propionibacterium as compared to the controls. Propionibacterium is probably derived from the skin and has lipolytic activity.34 We speculate that the higher relative abundance could be due to the increased amounts of lipids in the intestines, suggesting that these bacteria have colonized opportunistically when suitable energy sources were available.
Other studies have shown that nutrition affects the microbiota.12,13,14 Germ-free mice colonized with human microbiota showed extensive alterations in the microbiota when changing diets.12 Murine studies reported that omega-3 polyunsaturated fatty acids prevented dysbiosis caused by antibiotics35 and increased the Bifidobacterium population in the intestines.36 Furthermore, the microbiota may influence metabolism, for example, catabolism of nutrients, extraction of energy, and production of short chain fatty acids and vitamins.6,10 In malnourished children, poor nutrition affected normal microbiota and the subsequent altered microbiota composition caused poor growth.13 Mice colonized with microbiota from undernourished children show impaired growth, altered bone morphology, and metabolic abnormalities in the brain, muscle, and liver, as compared to mice colonized with healthy children’s microbiota.37
Studies have identified associations between intestinal microbiota and growth, but have focused on the development of obesity.27,38 There is limited knowledge about the relationship between microbiota and growth in preterm infants. We observed that gain in weight z-scores was associated with larger increase in relative abundance of Bifidobacterium. This indicates that infants with better growth had a more mature microbiota development.27 Bifidobacterium supplementation has been associated with improved growth among preterm infants who were treated with antibiotics.39 A recent study reported an association between early microbiota in preterm infants and weight gain in infancy.40 The authors found that Staphylococcus and Enterococcus were negatively associated with weight gain and that early levels of Enterobacter, Streptococcus, and Bacteroides were positively associated with weight gain at 1 month of age.40 Grier et al.28 investigated the relationship between nutrients, intestinal microbiota, and growth in term and preterm infants. The authors divided microbiota development into three successive phases and reported that the impact of nutrients on growth was affected by the microbiota phase.28 They also observed that increased nutrients appeared to promote transition of the microbiota into the next phase. Furthermore, poor growth was associated with delays in transitions from one phase to the next one.28
Infants on prolonged PN appeared to have delayed development of normal microbiota with reduced microbial diversity and richness, as well as more Enterococcus during the first weeks of life. We speculate that this could be due to the smaller human milk volumes provided for these infants emphasizing the importance of initiating human milk feeding if tolerated without delay. When adjusting for BW and sepsis, there were no longer differences between the PN groups. This could be due a strong association between low BW, increased risk of infection, and the need for prolonged PN. Also, low diversity and richness levels may be associated with increased risk of sepsis.
There are limitations to our study. Due to early termination of the study, the sample size was smaller than planned for. Thus, differences between the groups may not have been detected due to low power. Differences in baseline characteristics, particularly among the MVP infants, may have influenced the microbiota and concealed possible effects of the nutrients. The MVP intervention group had significantly lower mean BW, HC, BW z-scores, and HC z-score than the controls. Also, the infants in the intervention group had a higher rate of late-onset sepsis and tended to have more days on antibiotics as compared to the controls. The infection as well as the subsequent exposure to antibiotics may have affected the microbiota.16 Antibiotic use during the 5 days before sample collection was added as a covariate, but may not have eliminated the temporary effects on the microbiota.16 Despite attempts to correct for possible confounding factors, group differences may have had an effect on the intestinal microbiota that we were unable to adjust for in statistical tests. We did not collect information about maternal characteristics such as chorioamnionitis, antenatal antibiotic use, and maternal fever. These factors might affect the infants’ microbiota and subsequently confound the results. Towards the end of our study there were fewer fecal samples, particularly among the MVP controls. Thus, the results at the end of the study period should be evaluated with caution. Because the study included multiple nutrient interventions, we cannot conclude on associations between specific nutrients and microbiota composition. However, the aim of the intervention was to avoid selective nutrient deficiencies.
Conclusion
Microbial responses varied between individuals and few significant differences were detected when comparing the groups with different nutrient supplies. We observed that infants receiving enhanced nutrient supply maintained microbial richness levels better than infants receiving standard nutrient supply. MVP infants on enhanced nutrient supply showed a greater increase in microbial diversity as compared to MVP controls. Relative abundance of Bifidobacterium tended to increase more in MVP infants on standard nutrient supply as compared to MVP infants on enhanced nutrient supply. However, the lower relative abundance of Bifidobacterium in the MVP intervention group may be due to an increase in other microbes, indicated by the increase in diversity. Relative abundance of Staphylococcus appeared higher in the fecal samples collected early in EP infants with enhanced nutrient supply, and was followed by a more pronounced decrease as compared to EP controls. The nutrient intervention did not result in increased relative abundance of pathogenic microbes. Finally, infants who improved their weight z-scores appeared to have a more mature microbiota development, suggesting that there may be a link between improved growth and healthy microbiota.
References
Agostoni, C. et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 50, 85–91 (2010).
Horbar, J. D. et al. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000–2013. Pediatrics 136, e84–e92 (2015).
Sammallahti, S. et al. Infant growth after preterm birth and neurocognitive abilities in young adulthood. J. Pediatr. 165, 1109–1115 e1103 (2014).
Penders, J. et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118, 511–521 (2006).
Korpela, K. & de Vos, W. M. Early life colonization of the human gut: microbes matter everywhere. Curr. Opin. Microbiol. 44, 70–78 (2018).
Unger, S., Stintzi, A., Shah, P., Mack, D. & O'Connor, D. L. Gut microbiota of the very-low-birth-weight infant. Pediatr. Res. 77, 205–213 (2015).
Patel, A. L. et al. Longitudinal survey of microbiota in hospitalized preterm very-low-birth-weight infants. J. Pediatr. Gastroenterol. Nutr. 62, 292–303 (2016).
Shaw, A. G. et al. Late-onset bloodstream infection and perturbed maturation of the gastrointestinal microbiota in premature infants. PLoS ONE 10, e0132923 (2015).
Mai, V. et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE 6, e20647 (2011).
Groer, M. W., Gregory, K. E., Louis-Jacques, A., Thibeau, S. & Walker, W. A. The very low birth weight infant microbiome and childhood health. Birth Defects Res. C. 105, 252–264 (2015).
Sherman, M. P., Zaghouani, H. & Niklas, V. Gut microbiota, the immune system, and diet influence the neonatal gut–brain axis. Pediatr. Res. 77, 127–135 (2015).
Turnbaugh, P. J. et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 1, 6ra14 (2009).
Kane, A. V., Dinh, D. M. & Ward, H. D. Childhood malnutrition and the intestinal microbiome. Pediatr. Res. 77, 256–262 (2015).
Johnson, C. L. & Versalovic, J. The human microbiome and its potential importance to pediatrics. Pediatrics 129, 950–960 (2012).
Moltu, S. J. et al. Enhanced feeding and diminished postnatal growth failure in very-low-birth-weight infants. J. Pediatr. Gastroenterol. Nutr. 58, 344–351 (2014).
Korpela, K. et al. Intestinal microbiota development and gestational age in preterm neonates. Sci. Rep. 8, 2453 (2018).
Skjaerven, R., Gjessing, H. K. & Bakketeig, L. S. Birthweight by gestational age in Norway. Acta Obstet. Gynecol. Scand. 79, 440–449 (2000).
Salonen, A. et al. Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J. Microbiol. Methods 81, 127–134 (2010).
Korpela, K. mare: Microbiota Analysis in R Easily. R package version 1.0. 2016. https://github.com/katrikorpela/mare.
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Chernikova, D. A. et al. The premature infant gut microbiome during the first 6 weeks of life differs based on gestational maturity at birth. Pediatr. Res. 84, 71–79 (2018).
Stewart, C. J. et al. Temporal bacterial and metabolic development of the preterm gut reveals specific signatures in health and disease. Microbiome 4, 67 (2016).
Warner, B. B. et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case–control study. Lancet 387, 1928–1936 (2016).
Madan, J. C. et al. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch. Dis. Child Fetal Neonatal Ed. 97, F456–F462 (2012).
Hosny, M., Cassir, N. & La Scola, B. Updating on gut microbiota and its relationship with the occurrence of necrotizing enterocolitis. Hum. Microbiome J. 4, 14–19 (2017).
Dogra, S. et al. Rate of establishing the gut microbiota in infancy has consequences for future health. Gut Microbes 6, 321–325 (2015).
Grier, A. et al. Impact of prematurity and nutrition on the developing gut microbiome and preterm infant growth. Microbiome 5, 158 (2017).
Karlsson, C. L., Molin, G., Cilio, C. M. & Ahrne, S. The pioneer gut microbiota in human neonates vaginally born at term—a pilot study. Pediatr. Res. 70, 282–286 (2011).
Kaufman, D. & Fairchild, K. D. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin. Microbiol. Rev. 17, 638–680 (2004).
Wang, S., Hibberd, M. L., Pettersson, S. & Lee, Y. K. Enterococcus faecalis from healthy infants modulates inflammation through MAPK signaling pathways. PLoS ONE 9, e97523 (2014).
Stewart, C. J. et al. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatr. 101, 1121–1127 (2012).
Zhou, Y. et al. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: a case–control study. PLoS ONE 10, e0118632 (2015).
Whiteside, J. A. & Voss, J. G. Incidence and lipolytic activity of Propionibacterium acnes (Corynebacterium acnes group I) and P. granulosum (C. acnes group II) in acne and in normal skin. J. Invest. Dermatol. 60, 94–97 (1973).
Kaliannan, K., Wang, B., Li, X. Y., Bhan, A. K. & Kang, J. X. Omega-3 fatty acids prevent early-life antibiotic exposure-induced gut microbiota dysbiosis and later-life obesity. Int. J. Obes. (Lond.) 40, 1039–1042 (2016).
Patterson, E. et al. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br. J. Nutr. 111, 1905–1917 (2014).
Blanton, L. V. et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 351 (2016).
Scheepers, L. E. et al. The intestinal microbiota composition and weight development in children: the KOALA Birth Cohort Study. Int. J. Obes. (Lond.) 39, 16–25 (2015).
Mohan, R. et al. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr. Res. 64, 418–422 (2008).
Arboleya, S. et al. Intestinal microbiota and weight-gain in preterm neonates. Front. Microbiol. 8, 183 (2017).
Acknowledgements
This work was supported by the University of Oslo, the South-Eastern Norway Regional Health Authority, the Research Council of Norway, the Norwegian Foundation for Health and Rehabilitation, the Johan Throne Holst Foundation for Nutrition Research, and the Freia Medical Fund. This work was also supported by the SIAM Gravity Grant 024.002.002 of the Netherlands Organization for Scientific Research and Grants 137389, 141140, and 1272870 of the Academy of Finland.
Author information
Authors and Affiliations
Contributions
E.W.B.: Conceptualized and designed the study, included participants, acquired, analyzed growth/clinical characteristics data, interpreted growth and microbiota data, and drafted and reviewed the manuscript. K.K.: Conceptualized and designed study method for bioinformatical analyses of the microbiota and interpreted the microbiota data. Drafted, reviewed, and approved the manuscript. S.L.: Biostatistical analyses and interpretations of the microbiota data. Drafted, reviewed, and approved the manuscript. B.N.: Obtained grant money. Conceptualized and designed the study, included participants, interpreted data, and reviewed and approved the manuscript. S.J.M.: Conceptualized and designed the study, included participants, and reviewed and approved the manuscript. K.S.: Conceptualized and designed the study, included participants, and reviewed and approved the manuscript. A.E.R.: Conceptualized and designed the study, included participants, and reviewed and approved the manuscript. K.B.: Conceptualized and designed the study, included participants, and reviewed and approved the manuscript. P.O.I.: Obtained grant money, conceptualized and designed the study, interpreted data, and reviewed and approved the manuscript. W.M.d.V.: Conceptualized and designed the study method for analyzing the microbiota. Analyzed and interpreted the microbiota data, and reviewed and approved the manuscript. C.A.D.: Obtained grant money, conceptualized and designed the study, analyzed and interpreted data, and reviewed and approved several versions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.J.M. has during the past 2 years received payment/honorarium for lectures/consultations from Baxter. The remaining authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Blakstad, E.W., Korpela, K., Lee, S. et al. Enhanced nutrient supply and intestinal microbiota development in very low birth weight infants. Pediatr Res 86, 323–332 (2019). https://doi.org/10.1038/s41390-019-0412-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0412-x