Abstract
Background
Subgaleal hemorrhage (SGH) is reported to be associated with severe hemodynamic instability, coagulopathy, and even mortality. The importance of the presence or absence of neonatal encephalopathy in predicting SGH outcomes has not been explored. The aim of this study was to determine the relationship of clinical encephalopathy to short-term outcomes in neonates with SGH.
Methods
Neonates ≥35 weeks gestation, diagnosed radiologically with SGH between 2010 and 2017, were included. Cases were divided into encephalopathic and non-encephalopathic. Demographic, clinical, and outcome data were compared between groups.
Results
Of 54,048 live births, 56 had SGH, of them 13 (23%) had encephalopathy. When compared to the non-encephalopathic neonates, encephalopathic neonates had lower Apgar scores, lower hemoglobin, lower platelet count, longer neonatal intensive care unit stay, two (15%) deaths, and four (31%) required blood transfusion. No non-encephalopathic infant with SGH died or required blood transfusion. Notably, on magnetic resonance imaging (MRI), a majority of subgaleal collections had either no or minimal blood products.
Conclusions
In the absence of encephalopathy, SGH is not associated with adverse short-term outcome. Neurological assessment is likely to identify infants at higher risk for adverse outcome. The absence of MRI signal consistent with blood in subgaleal collection warrants further research.
Similar content being viewed by others
Introduction
Subgaleal hemorrhage (SGH) is described as bleeding in the loose connective tissue between the skull and the galea aponeurotica.1 SGH is classically characterized by a diffuse, gravity-dependent, fluctuant scalp mass that crosses suture lines and can progress to cause eye lid swelling and ear displacement. Although it can occur following spontaneous delivery and cesarean section, SGH is a known complication of instrumental delivery, particularly vacuum delivery.2,3,4,5 The incidence of SGH with vacuum delivery is 3–7.6 per 1000 vacuum extractions, with some studies reporting incidences as high as 210 per 1000 vacuum extractions, while spontaneous vaginal deliveries have a lower reported incidence of 0.1–0.6 per 1000 deliveries.4,5,6,7,8,9,10,11,12 The complications of SGH include shock, coagulopathy and disseminated intravascular coagulation (DIC), anemia, renal or hepatic injury, lactic acidosis, and even death.12,13 Older prior studies reported mortality rates associated with SGH of 17–25%, but more recent studies have shown lower mortality rates of 5–14% in neonatal intensive care unit (NICU) admissions, including one study reporting a mortality rate of 2.8% with close monitoring and aggressive management.5,6,8,10,12,14 SGH has been associated with skull fractures, brain injury, intracranial hemorrhage, seizures, and hypoxic–ischemic encephalopathy (HIE).1,6,12,14 Because of the appreciable morbidity and mortality associated with SGH, multiple advisory reports from the USA, Canada, and Australia have focused on these risks from vacuum delivery.15 The importance of clinical encephalopathy in association with SGH has not been thoroughly examined or characterized. Thus, the aim of this study was to determine the relationship of clinical encephalopathy to short-term outcomes in newborn infants diagnosed radiologically with SGH.
Patients and methods
The study population included all infants diagnosed radiologically with SGH at Brigham and Women’s Hospital, Boston, over a 7-year period. This project has been approved by the Partners Human Research Committee and a waiver of informed consent was given. Patients were identified via key word search in an ultrasound reporting database or diagnosis search of electronic medical record between 1 January 2010 and 31 July 2017. Delivery information and neonatal hospital course were recorded. Only late pre-term or term neonates (gestational age ≥35 weeks) were included. Patients with known congenital brain abnormalities were excluded. Information recorded included demographics, gestational age, type of delivery (spontaneous vaginal, forceps, vacuum assisted, operative), maternal age, gravida status, birthweight, APGAR scores, physical exam, neonatal laboratory work, length of stay in the NICU, status at discharge, head ultrasound (HUS) findings, and magnetic resonance imaging (MRI) results, including presence, size, and location of hematomas. For a suspected diagnosis of SGH to be considered radiologically confirmed, the abnormality must have been reported on ultrasound, or MRI. Adverse short-term outcome has focused on the need of blood product transfusion and mortality. Cases were divided into two cohorts for analysis: those with neonatal encephalopathy, defined by clinical neurological criteria, and those without encephalopathy.
Diagnosis of neonatal encephalopathy
Patients were categorized as encephalopathic if they were described in the medical record to have either neonatal encephalopathy or seizures. Neonatal encephalopathy has been recently defined as “a clinically defined syndrome of disturbed neurologic function in the earliest days of life in an infant born at or beyond 35 weeks of gestation, manifested by a subnormal level of consciousness or seizures, and often accompanied by difficulty with initiating and maintaining respiration and depression of tone and reflexes.”16 In our institution, encephalopathy is defined by the presence of seizures or any abnormality in an abbreviated neurological examination, assessing six domains, namely level of consciousness, spontaneous activity, muscle tone, posture, primitive reflexes, or autonomic functions. In this study, neonatal encephalopathy at presentation has been defined as mild, moderate, or severe based on previously published standardized criteria.17
Magnetic resonance imaging
MRI data were derived from clinically acquired MRI images performed between days 1 and 7. The Neonatal Brain MRI Protocol at Brigham and Women’s Hospital includes the following sequences: axial T2 Turbo spin echo, sagittal motion mitigated magnetization-prepared rapid gradient echo, coronal T2, Ax diffusion tensor imaging, and axial susceptibility-weighted imaging. The pattern of hemorrhage in these different sequences is largely affected by the evolution of blood products over time. Intracranial and extracranial hemorrhages can be divided into hyperacute (<6 h), acute (6–72 h), early subacute (3–10 days), late subacute (1 week–months), and chronic (months–years).
Acute hemorrhage (6–72 h) is hypointense on T1W due to effect of T2 shortening. On T2W there is significant hypointensity starting at the periphery. Early subacute hemorrhage (days 3–10) presents as hyperintense on T1W sequences, and hypointense on T2W sequences. It also presents with susceptibility artifact of paramagnetic substances (hemosiderin) affecting the magnetic milieu “blooming” on SWI.18 A qualitative estimation of blood present in subgaleal collections relied on all three sequences.
Statistical analysis
Descriptive statistics are presented as proportions for categorical variables, and mean ± standard deviation (SD) or median (interquartile range) for continuous variables, unless otherwise noted. For continuous variables, differences between groups were evaluated using independent sample’s t tests (parametric) and Mann–Whitney U test (non-parametric). Paired t test was used to compare repeated measurements. For nominal variables, Fisher’s exact tests were used. Statistical analysis was performed using IBM SPSS Statistics (Version 24).
Results
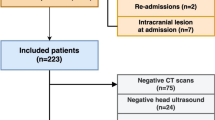
Of 54,048 live births, 87 infants had a diagnosis of SGH determined either by ultrasound or clinically. Of these, 32 cases were excluded due to pre-term delivery (n = 6), insufficient records (n = 8), lack of radiologically confirmed subgaleal collection (n = 17), and presence of known congenital brain anomaly (n = 1). Of the remaining 56 patients, 30 infants (54%) had instrumental delivery, principally vacuum extraction. Thirteen (23%) of the 56 had clinical neurological signs indicative of encephalopathy, 11 of them were moderate and 2 were mild. Of these 13 patients, 3 had electroclinical seizures, 2 had subclinical seizures, and 2 had clinical seizures not confirmed by electroencephalogram (EEG). Of the non-encephalopathic group (n = 43), 35 neonates (81%) presented solely with scalp swelling or increased head circumference (HC) following instrumental delivery. Comparing the 13 neonates with encephalopathy to the 43 without, there was no difference in gestational age, maternal age, maternal body mass index (BMI), rate of instrumental delivery, or delivery mode (Table 1). However, encephalopathic infants had lower median (interquartile range (IQR)) Apgar scores at 1 min (5 (2.5–7) vs. 7 (4.5–8), p = 0.029) and at 5 min (7 (6.5–8) vs. 9 (8–9), p = 0.03). Encephalopathic infants had lower hemoglobin concentration (12.2 ± 3.1 vs. 15.3 ± 2.3 g/dl, p = 0.004), hematocrit (33.7 ± 8.1 vs. 42.1 ± 6.1%, p < 0.001), and platelet count (144 ± 71 × 109 vs. 190 ± 58.4 × 109 per liter, p = 0.027). Infants with neonatal encephalopathy had longer median (IQR) NICU stay (10 (8.5–14.5) vs. 3 (1–4), p < 0.001).
No infant without encephalopathy died or required blood transfusion. In contrast, among the 13 infants with clinical encephalopathy, there were two deaths (15.4%), four infants (30.8%) required packed red blood cell transfusions due to dropping hematocrit, and of these four, two also received fresh-frozen plasma for abnormal coagulation profile.
Of the 13 infants in the clinical encephalopathic group, 8 presented with encephalopathy requiring therapeutic hypothermia. These 13 infants displayed parenchymal abnormalities on their brain MRI/HUS, predominantly findings consistent with hypoxic–ischemic cerebral injury. Other abnormalities detected included deep medullary vein thrombosis, venous infarctions, and punctate hemorrhages. One infant had germinal matrix cysts as the only abnormalities. The details of these 13 encephalopathic patients are summarized in Table 2.
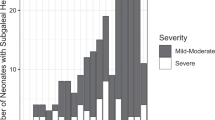
HC was measured on admission and repeated through the hospital admission period. Only 28 patients had two measurements in the first 48 h: 10 from the encephalopathic group and 18 from the non-encephalopathic group. Using paired t test, in all patients, HC increased from 34.1 ± 1.9 to 34.6 ± 2 cm (p = 0.022). However, when this difference was examined in each group, non-encephalopathic patients had an increase in their HC from 33.9 ± 2 to 34.6 ± 2.3 cm (p = 0.03), while the encephalopathic patients had a stable HC (34.3 ± 1.8 vs. 34.7 ± 1.4, p = 0.356). These changes are demonstrated in Fig. 1a, b.
In this study, SGH was diagnosed either by HUS or MRI. MRI data were available for 17 infants, 12 of them were in the encephalopathic group. Timing of the MRI was 2.8 ± 1.4 days old (3.1 ± 1.2 days for encephalopathic and 1.9 ± 0.9 days for non-encephalopathic). One infant diagnosed with SGH by ultrasound (US) did not exhibit any traces of subgaleal collection on MRI. The content of the subgaleal collections was evaluated using T1, T2, and SWI sequences. More than half of the subgaleal collections (10/16) did not show any evidence of blood products, while the rest of the infants showed either trace (three patients) or small amounts (three patients) of blood products. Details of the MRI findings in these 17 studies are described in Table 3. Figures 2 and 3 demonstrate HUS and MRI findings in two study patients.
Axial reformations of a sagittal magnetization-prepared rapid gradient echo (MPRAGE) (T1 weighted) (a), axial T2 Turbo spin echo (TSE) (b), and axial susceptibility-weighted image or SWI (c) show small bilateral subgaleal collections (*) with slightly increased T1 signal, isointense T2 signal but minimal blooming, suggesting minimal blood products. Coronal T2 TSE (d) in the same patient shows a right cephalohematoma (thick white arrow) and a subgaleal collection that was bright on T2. This collection was isointense on T1 and did not bloom. e, f demonstrated corresponding ultrasound images. Notice that the cephalohematoma does not cross the suture line, while the subgaleal collection does (study case # 69)
Axial (a) and coronal (d) reformations of a sagittal magnetization-prepared rapid gradient echo (MPRAGE) (T1 weighted) as well as axial T2 Turbo spin echo (TSE) (b) and axial susceptibility-weighted image or SWI (c) show a small right cephalohematoma with blood products (thick white arrow) that are bright on T1, dark on T2, and bloom on SWI. In comparison, the large subgaleal collection (*) is predominately serous fluid without significant blood products. Also of note is a small amount of layering blood in the subcutaneous space (black arrow). e, f demonstrate corresponding ultrasound with small right cephalohematoma (thick white arrow) and large subgaleal collection (*) (study case # 38)
Discussion
This study is the largest reported case series describing radiologically confirmed SGH in the newborn infant. In this report, we identified the presence of clinical encephalopathy as a clinical biomarker for adverse outcome for newborns with SGH. Changes in HC were not contributory. In addition, this report is the first to describe absence/paucity of blood products in most subgaleal collections in the neonatal period.
For infants with radiologically confirmed SGH, we found a 7% morbidity manifested by need for blood transfusion and a 3.6% mortality. However, these complications were solely confined to the encephalopathy group. Moreover, mortality was related to redirection of care in the presence of poor prognosis. These findings contrast with older reports of SGH morbidity and mortality related to hemodynamic instability, shock, and DIC.5,7,10 While morbidity and mortality are not unexpected in the setting of neonatal encephalopathy, this report demonstrates the absence of adverse short outcome in non-encephalopathic patients diagnosed with SGH.
The association of subgaleal collection with encephalopathy was previously reported in multiple series of clinically diagnosed SGH. Although an older study reported that HIE was present in 73% of SGH cases,14 more recent and larger reports showed this association in only 10% of SGH cases.5 Since HIE can be associated with hemodynamic instability, shock, DIC, and parenchymal hemorrhage, it is possible that reported complications with SGH might not be directly related to the SGH per se, but rather to the associated HIE.
Earlier reports recommended serial head measurements and classified SGH based on head size.14 This practice continues to be common in the NICU, and classification systems using changes in head size have been recommended.15 Our unit has adopted a protocol of serial HC measurements following any instrumental delivery. In the current study population, changes in HCs were not helpful to differentiate the encephalopathic babies with unfavorable outcome from non-encephalopathic infants with favorable outcome. In contrast, the presence of encephalopathy itself was a key predictor of adverse outcome. Of note, four of the encephalopathic infants did not have report of scalp swelling by clinical examination in their records. This could be related to under-documentation or presence of small collections.
We demonstrate for the first time that most neonatal subgaleal collections considered to be SGH are often either non-hemorrhagic or contain very small amounts of blood products. Suggested mechanisms proposed for SGH included suture diastasis, fracture of the skull, and ruptured emissary vein secondary to fragmentation of the superior margin of the parietal bone. Such mechanisms are exacerbated in the presence of bleeding tendencies, such as hemophilia and vitamin K deficiency.1 However, non-hemorrhagic subgaleal fluid collections have been reported to occur weeks to months after delivery.19,20,21,22,23 Mechanisms proposed for these later collections have included small SGHs, which stimulate vascular exudate, lymphatic drainage defect, complication of scalp electrodes, or even a CSF leak. In the largest reported series of 11 infants who presented weeks after birth, all had a history of vacuum delivery or attempted vacuum delivery at birth.24 This finding raises the possibility that the mechanism for the later development of a fluid collection is similar to that for neonatal SGH, but of lesser severity.
In this study, SGH was diagnosed mainly by HUS. MRI data were available in 12/13 encephalopathic and in 5/43 non-encephalopathic patients. While HUS interpretations commonly conclude that these collections are SGH, it is very difficult by HUS to be confident about the nature of this fluid. On MRI, bleeding during parturition would be expected to show fluid with decreased signal on T2 that “bloomed” on SWI and increased T1 signal beginning ~2–3 days after birth. None of these findings were prominent in our MRI scans. Because of the lack of consistency in MRI imaging in previously published reports of neonatal SGH, it is difficult to define the nature of the subgaleal fluid collections as hemorrhage or more complex exudates or both in these reports.
Limitations of this study include its retrospective nature, selection criteria by medical records search, which may have missed cases if not properly documented, and inclusion of only cases with a radiological diagnosis of SGH either by HUS or MRI, which could include incidental collections of no clinical significance. Most neonates who received MRI were in the encephalopathic group, which allowed proper identification of the nature of the subgaleal collection, while most non-encephalopathic neonates were solely diagnosed by HUS. There was also under-documentation and non-standardization of repeated HC measurements, which may make these estimates less reliable. Finally, there were no systematic neurodevelopmental follow-up data available for these cases.
Conclusions
In our retrospective cohort, in the absence of encephalopathy, SGH is not associated with adverse short-term outcome. Detailed neurological assessment after birth to detect signs of neonatal encephalopathy will identify those neonates with SGH at higher risk for adverse outcome. Absent to trace amounts of blood in SGH by MRI warrant further research and would suggest that “subgaleal collection” may be a better terminology. MRI might help identifying those with associated brain injury or those with hemorrhagic subgaleal collections who are likely at risk for greater complications.
References
Govaert, P., Vanhaesebrouck, P., De Praeter, C., Moens, K. & Leroy, J. Vacuum extraction, bone injury and neonatal subgaleal bleeding. Eur. J. Pediatr. 151, 532–535 (1992).
Ahuja, G. L., Willoughby, M. L. N., Kerr, M. M. & Hutchison, J. H. Massive subaponeurotic haemorrhage in infants born by vacuum extraction. BMJ 3, 743–745 (1969).
Williams, M. F., Jacobs, M. & Moosa, A. Subaneurotic haemorrhage of the newborn. S. Afr. Med. J. 52, 176–178 (1977).
Uchil, D. & Arulkumaran, S. Neonatal subgaleal hemorrhage and its relationship to delivery by vacuum extraction. Obstet. Gynecol. Surv. 58, 687–693 (2003).
Boo, N. Y., Foong, K. W., Mahdy, Z. A., Yong, S. C. & Jaafar, R. Risk factors associated with subaponeurotic haemorrhage in full-term infants exposed to vacuum extraction. BGOJ Int. J. Obstet. Gynaecol. 112, 1516–1521 (2005).
Plauché, W. C. Subgaleal hematoma: a complication of instrumental delivery. JAMA 244, 1597–1598 (1980).
Ng, P., Siu, Y. & Lewindon, P. Subaponeurotic haemorrhage in the 1990s: a 3‐year surveillance. Acta Pædiatr. 84, 1065–1069 (1995).
Gebremariam, A. Subgaleal haemorrhage: risk factors and neurological and developmental outcome in survivors. Ann. Trop. Paediatr. 19, 45–50 (1999).
Christensen, R. D., Baer, V. L. & Henry, E. Neonatal sub-galeal hemorrhage in a multihospital healthcare system: prevalence, association and outcomes. Eur. J. Neonatol. Res. 1, 4–11 (2011).
Swanson, A. E., Veldman, A., Wallace, E. M. & Malhotra, A. Subgaleal hemorrhage: risk factors and outcomes. Acta Obstet. Gynecol. Scand. 91, 260–263 (2012).
Boo, N. Y. Subaponeurotic haemorrhage in Malaysian neonates. Singap. Med. J. 31, 207–210 (1990).
Chang, H.-Y. et al. Neonatal subgaleal hemorrhage: clinical presentation, treatment, and predictors of poor prognosis. Pediatr. Int. 49, 903–907 (2007).
Adedoyin, O. T., AWBR, Johnson, Mokuolu, O. A. & Ajayi, O. A. Acute renal failure complicating neonatal sub-galeal hemorrhage [3]. Pedia. Nephrol. 18, 848–849 (2003).
Chadwick, L. M., Pemberton, P. J. & Kurinczuk, J. J. Neonatal subgaleal haematoma: associated risk factors, complications and outcome. J. Paediatr. Child Health 32, 228–232 (1996).
Colditz, M. J., Lai, M. M., Cartwright, D. W. & Colditz, P. B. Subgaleal haemorrhage in the newborn: a call for early diagnosis and aggressive management. J. Paediatr. Child Health 51, 140–146 (2015).
2014 Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet. Gynecol. 123, 896–901 (2014).
Shankaran, S. et al. Evolution of encephalopathy during whole body hypothermia for neonatal hypoxic–ischemic encephalopathy. J. Pediatr. 160, 567–572 e563 (2012).
Naidich, T. P. Imaging of the Brain pp xv, 1052pp (Saunders/Elsevier, Philadelphia, 2013).
Lee, J. J. & Wenger T. L. Delayed subaponeurotic fluid collections of infancy. J. Pediatr 197, 310–310.e1 (2018).
Wang, S., Drake, J. & Kulkarni, A. V. Management and outcome of spontaneous subaponeurotic fluid collections in infants: the Hospital for Sick Children experience and review of the literature. J. Neurosurg. Pediatr. 18, 442–447 (2016).
Hopkins, R. E., Inward, C., Chambers, T. & Grier, D. Sub-aponeurotic fluid collections in infancy. Clin. Radiol. 57, 114–116 (2002).
Petraglia, A. L., Moravan, M. J., Marky, A. H. & Silberstein, H. J. Delayed sub-aponeurotic fluid collections in infancy: three cases and a review of the literature. Surg. Neurol. Int. 1, 34 (2010). Available from: https://surgicalneurologyint.com/surgicalint-articles/delayed-sub-aponeurotic-fluid-collections-in-infancy-three-cases-and-a-review-of-the-literature/
Schoberer, A. et al. Sub-aponeurotic fluid collections: a delayed-onset self-limiting cerebrospinal fluid fistula in young infants. Eur. J. Paediatr. Neurol. 12, 401–403 (2008).
Smith, A. et al. Delayed infant subaponeurotic (subgaleal) fluid collections: a case series of 11 infants. J. Emerg. Med. 50, 881–886 (2016).
Author information
Authors and Affiliations
Contributions
M.E.-D. had substantial contributions to conception and design, data analysis, and interpretation of data. He also drafted the article and had final approval of the version to be published. M.P. had substantial contributions to conception and design, acquisition of data, analysis, and interpretation of data. She co-drafted the article and had final approval of the version to be published. L.J. had substantial contributions to conception and design, acquisition, and interpretation of data. She contributed to revising it critically for important intellectual content, and had final approval of the version to be published. C.B.B. had substantial contributions to conception and design, acquisition, and interpretation of data. She contributed to revising it critically for important intellectual content, and had final approval of the version to be published. P.E.G. had substantial contributions to conception and design, acquisition, and interpretation of data. She contributed to revising it critically for important intellectual content, and had final approval of the version to be published. J.R. had substantial contributions to conception and interpretation of data. He contributed to revising it critically for important intellectual content, and had final approval of the version to be published. J.J.V. had substantial contributions to conception and interpretation of data. He contributed to revising it critically for important intellectual content, and had final approval of the version to be published. T.I. had substantial contributions to conception and interpretation of data. She contributed to revising it critically for important intellectual content, and had final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Dib, M., Parziale, M., Johnson, L. et al. Encephalopathy in neonates with subgaleal hemorrhage is a key predictor of outcome. Pediatr Res 86, 234–241 (2019). https://doi.org/10.1038/s41390-019-0400-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0400-1
This article is cited by
-
Unconjugated bilirubin is correlated with the severeness and neurodevelopmental outcomes in neonatal hypoxic-ischemic encephalopathy
Scientific Reports (2023)
-
Neonatal subgaleal hemorrhage: twenty years of trends in incidence, associations, and outcomes
Journal of Perinatology (2023)