Abstract
Background
Serum bilirubin levels are inversely associated with obesity in adults. We are interested in whether neonatal jaundice is associated with childhood obesity in preterm infants.
Methods
Data were obtained from the US Collaborative Perinatal Project. Neonatal bilirubin levels were used as exposure factors for obesity at age 7 years. Logistic regression models were used to control for potential confounders and calculate odds ratios (ORs). A generalized estimating equation (GEE) model was used to correct for intracluster correlation coefficient. SAS was used for all statistical analyses.
Results
In the study subjects, 865 of 5019 preterm infants were obese at age 7 years. While neonatal total serum bilirubin (TSB) rose 1 mg/dl, body mass index (BMI) increased 0.03 kg/m2 (95% confidence interval (CI) 0.02, 0.04). Compared with infants with TSB <3 mg/dl, the ORs (95% CIs) for obesity in infants with 3 mg/dl≤ TSB <6 mg/dl, 6 mg/dl≤ TSB <9 mg/dl, 9 mg/dl≤ TSB <12 mg/dl and TSB ≥12 mg/dl were, respectively, 1.18 (0.87, 1.59), 1.25 (0.93, 1.67), 1.52 (1.11, 2.09), and 1.67 (1.22, 2.07). By using subtypes of bilirubin as exposure factors and the GEE model to correct for intracluster correlation coefficient, similar trends of associations were observed.
Conclusion
Neonatal bilirubin levels have positive trends of associations with childhood obesity in preterm infants.
Similar content being viewed by others
Introduction
The obesity epidemic has increased dramatically over the past four decades and has posed a considerable public health threat worldwide. The prevalence of obesity has grown from approximately 3% of men and 6% of women in 1975 to 11% of men and 15% of women in 2014.1 It is a major contributor to the diabetes epidemic and is a risk factor for other chronic diseases and disabilities.1 At the same time, approximately 41 million children under the age of 5 years were overweight or obese in 2016.2 The International Obesity Task Force has shown that 10% of school-aged (5–17 years) children are estimated to be overweight, and of these overweight children, a quarter are obese.3 Childhood obesity epidemics can continue into adulthood, greatly increasing the morbidity of obesity-related conditions and medical treatments in later life, which will contribute to greater health service costs and losses to society.
Obesity is characterized by enhanced oxidative stress4 and low-grade systemic inflammation.5 A previous study found that bilirubin is a potent endogenous antioxidant and cytoprotectant with anti-inflammatory properties.4 The physiological level of serum bilirubin has an effect on preventing oxidative stress-related diseases.6 Several population-based studies have reported an inverse relationship between the concentration of bilirubin and obesity.7,8 However, except for several animal model studies,9,10 all of these population-based studies were performed in subjects with physiological levels of bilirubin. To date, there has been no evidence of exposure to high levels of bilirubin related to this topic.
Neonatal jaundice is a common phenomenon characterized by a much higher level of serum bilirubin than in any other period of life, and preterm newborns are more vulnerable to suffering from neonatal jaundice than are term newborns.11 In addition, bilirubin has toxic effects on developing neuronal tissues, and a high level of serum bilirubin is associated with neurological dysfunction in newborn babies.12,13 Thus, our major area of interest is whether neonatal jaundice is associated with childhood obesity in preterm infants. In a previous study of neonatal jaundice and its long-term effect on children with asthma, researchers found that phototherapy for jaundice may be an influencing factor and thus confound the conclusion.14 To exclude the effect of phototherapy, we used the US Collaborative Perinatal Project (CPP) data, which recruited participants before phototherapy was routinely used for the treatment of neonatal jaundice, to investigate the correlation between the neonatal serum bilirubin levels and childhood obesity.
Methods
Study population
The CPP was a large prospective birth cohort study performed in the United States from 1959 to 1965 among 46,021 women with 56,990 pregnancies at 12 centers. During the study period, weight and height were measured and recorded by a trained pediatrician using standardized procedures at each follow-up visit. Other maternal and infant characteristics were documented in the study questionnaire, and a detailed description of the study has been provided elsewhere.15 The CPP data are publicly available through the US National Archives (www.archives.gov/). The use of publicly available anonymous data is exempt from our Institutional Review Board review.
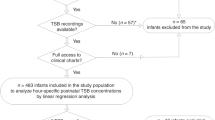
A total of 2195 stillbirths or abortions were initially excluded from this analysis. We restricted the study population to singletons, and 1148 multiple births were excluded. Because the study subjects were preterm infants and because most babies with gestational age <28 weeks (n = 782) had inaccurate data on gestational age in the CPP, we excluded births with gestational age at birth <28 weeks, ≥37 weeks (n = 44,797), or missing (n = 41). Infants with unknown birth weight (n = 224), neonatal bilirubin level (n = 758), or body mass index (BMI) at age 7 years (n = 1584) were also excluded. Finally, after infants with severe congenital structural anomalies were excluded (n = 442), 5019 preterm infants remained for analysis (Fig. 1).
Bilirubin measurement
Total serum bilirubin (TSB) measurements were performed by the diazo method in 11 of the 12 centers and by direct spectrophotometry in the remaining center. The coefficient of variation for standard specimens among CPP laboratories was approximately 10%, and the intralaboratory coefficient of variation was approximately 2%.16 Although less than ideal, this degree of reliability is similar to that reported in a survey of bilirubin measurements 30 years later.17 TSB measurements were obtained for all neonates between 36 and 60 h after delivery (as close as possible to 48 h after delivery) and were repeated 24 h later if the TSB level was higher than 10 mg/dl (171 μmol/l). If the second TSB level still exceeded 10 mg/dl, a third measurement was required 4 to 5 days later, with additional bilirubin measurements obtained at the discretion of physicians at each study site. We used the maximum TSB level measured for each neonate as the exposure factor and categorized the neonates into five groups based on the levels of maximum TSB concentrations: TSB <3 mg/dl, 3 mg/dl≤ TSB <6 mg/dl, 6 mg/dl≤ TSB <9 mg/dl, 9 mg/dl≤ TSB <12 mg/dl, and TSB ≥12 mg/dl. In addition, a total of 2252 infants had neonatal serum-conjugated bilirubin (CB) values. We further used serum CB and serum-unconjugated bilirubin (UCB) as exposure factors to explore the associations between the different types of bilirubin and childhood obesity.
Outcome and confounders
BMI at age 7 years was calculated from children’s weight and height. Obesity was primarily defined as BMI at or above the 95th percentile for children of the same age and sex based on the CDC growth chart of the United States.18 Some studies have used different BMI cut points to define obesity. Second, we used the World Health Organization reference (BMI ≥+2 SD) to define obesity to test the robustness of our analysis.19 Perinatal factors were chosen as potential confounders: maternal characteristics included age at delivery (<20, 20–34, and ≥35 years), marital status (married, unmarried, and other), maternal smoking (0, 1–19, and 20 cigarettes per day during pregnancy), hypertensive disorders during pregnancy (none, moderate, and severe), socioeconomic status (comprised of five categories as assessed by the original CPP investigators), maternal pregnancy BMI (<18.5, 18.5–25, and >25 kg/m2), and BMI gain during pregnancy (<3, 3–6, and >6 kg/m2). Infant characteristics included race (white, black, and other race), sex (male and female), gestational age (categorical variable), delivery method (vaginal, cesarean section, and others), birth weight (<2500 and ≥2500 g), and feeding method (exclusively breast, exclusively bottle, mixed feeding, and unknown).
Statistics analysis
We used the Cochran–Mantel–Haenszel χ2 test to assess the differences in maternal and infant baseline characteristics among neonates with different levels of maximum TSB concentrations. The linear coefficients between neonatal TSB levels and BMI at age 7 years were calculated first. Second, obesity at age 7 years was defined as a dichotomous variable. A univariate logistic regression model (model 1) was used to calculate the crude odds ratios (ORs) for obesity at age 7 years in each group relative to the infants with maximum TSB <3 mg/dl. To adjust for potential confounders, a multivariate logistic regression model (model 2) was performed to calculate the adjusted ORs (aORs). Because mothers were the primary sampling unit of the CPP, approximately 15% of mothers had more than 1 infant enrolled, and children from the same family shared similar genes and a similar household environment; thus, we ran a generalized estimating equation (GEE) model (model 3) to correct for intracluster correlation coefficient. SAS version 9.2 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Maternal and infant baseline characteristics for preterm neonates with different concentrations of maximum TSB are shown in Table 1. There were significant differences in birth weight, race, gestational age, feeding method, maternal age, maternal smoking, socioeconomic status, and BMI gain during pregnancy among preterm babies with different levels of TSB concentrations, and there were no significant differences in sex and maternal BMI before pregnancy.
There were 865 obese children aged 7 years among the 5019 preterm babies, with an overall incidence of 17.2%. The linear regression model shows that as neonatal TSB rose 1 mg/dl, BMI increased 0.03 kg/m2 (95% CI 0.02, 0.04) in our study subjects. Table 2 and Fig. 2 show that in preterm babies, relative to the group with maximum neonatal TSB <3 mg/dl, the aORs for obesity at age 7 years in babies with 3 mg/dl≤ TSB <6 mg/dl, 6 mg/dl≤ TSB <9 mg/dl, 9 mg/dl≤ TSB <12 mg/dl, TSB ≥12 mg/dl were, respectively, 1.18 (95% CI 0.87, 1.59), 1.25 (95% CI 0.93, 1.67), 1.52 (95% CI 1.11, 2.09), and 1.67 (95% CI 1.22, 2.07). Similar results were obtained by using the GEE model to correct for intracluster correlation coefficient.
Table 3 shows the associations between different types of neonatal serum bilirubin and obesity in preterm babies at age 7 years. Relative to the group with UCB <3 mg/dl, the aORs of obesity at age 7 years were 1.07 (95% CI 0.70, 1.63), 1.29 (95% CI 0.86, 1.93), 1.54 (95% CI 0.99, 2.38), and 1.65 (95% CI 1.08, 2.51) among babies with, respectively, 3 mg/dl≤ UCB <6 mg/dl, 6 mg/dl≤ UCB <9 mg/dl, 9 mg/dl≤ UCB <12 mg/dl, and UCB ≥12 mg/dl. Meanwhile, compared with babies with serum CB <1 mg/dl, the aORs of obesity at age 7 years were 1.22 (95% CI 0.89, 1.67) and 1.46 (95% CI 0.88, 1.41) among babies with 1 mg/dl≤ CB <2 mg/dl and CB ≥2 mg/dl, respectively. After corrections were made for the intracluster correlation coefficient in model 3, the results were similar.
With BMI ≥+2 SD being used to define obesity, relative to the group with TSB <3 mg/dl, the aORs for obesity at age 7 years in babies with 3 mg/dl≤ TSB <6 mg/dl, 6 mg/dl≤ TSB <9 mg/dl, 9 mg/dl≤ TSB <12 mg/dl, and TSB ≥12 mg/dl were, respectively, 1.18 (95% CI 0.72, 1.94), 1.29 (95% CI 0.80, 2.27), 1.32 (95% CI 0.79, 2.23), and 1.75 (95% CI 1.06, 2.89). Similar results can be seen in model 3 (Supplemental Table S1).
Discussion
In the present study, we first report a positive association between neonatal serum bilirubin and childhood obesity in preterm infants. Even though only groups with extremely high levels of TSB reached statistical significance, a trend was still observed that a higher level of neonatal serum bilirubin was associated with a higher risk of obesity. In addition, our results showed that the two subtypes of bilirubin and TSB had similar trends of association with childhood obesity in preterm infants. This finding was consistent with that of a previous study20 reporting that TSB, CB, and UCB all have similar associations with metabolic syndrome.
Previous studies have reported that serum bilirubin had an anti-inflammatory effect and could reduce obesity-related oxidative stress and systemic inflammation.4,5 In some studies, the anti-inflammatory role of bilirubin was used to interpret the inverse relationship between the concentrations of bilirubin and obesity in adults with physiological levels of serum bilirubin.7,8 For our vastly different results from these studies in adults, we suspect that different potential mechanisms should exist in preterm infants. It is generally believed that given their exposure to extremely high levels of bilirubin, neonatal infants are at an increased risk of bilirubin-induced neurotoxicity. Due to the high affinity for lipids of UCB, the main form of neonatal serum biirubin, it has neurotoxicity and induces cerebral palsy and other kinds of neurodevelopmental disabilities.21 The high level of UCB exceeds the binding capacity of serum albumin, and the unbinding UCB can cross the blood–brain barrier. Given their immature blood–brain barrier and the low bilirubin binding capacity of serum albumin, premature infants have higher risks of bilirubin-induced neurotoxicity than do term infants.22 In the present study, preterm infants with TSB or UCB ≥12 mg/l were more likely to have neurodevelopmental disabilities (Supplemental Table S2).
Intuitively, children with neurodevelopmental disabilities often engage in less physical exercise and have more behavioral problems (e.g., lack of control in their regulation of food intake, persistent short sleep).21,23,24 Furthermore, mothers of infants with neurodevelopmental disabilities are usually under high stress and are more likely to have a child identified as obese.25 These factors could partly indicate that the risks of obesity and neurodevelopmental disabilities increase simultaneously in infants with serum bilirubin ≥12 mg/l in the present study. However, even though no statistical significance existed, a positive trend was still observed that a higher level of neonatal serum bilirubin was associated with a higher risk of obesity in infants with serum bilirubin <12 mg/dl. Thus, we believe that, except for bilirubin-induced neurotoxicity, other potential mechanisms might be discovered in the future that clarify the phenomenon of the positive trend of associations between neonatal serum bilirubin and childhood obesity.
Our study has some strengths and limitations. A major strength of our study was the large nationwide study sample collected over 7 years. All data in our study, with the exception of self-reported smoking, were prospectively collected and evaluated by the objective assessment of medical records and laboratory tests, thus ensuring that our data were not subject to recall bias. Second, in the CPP study period (1959–1965), phototherapy was not yet routinely used, as it was proven to have confounding effects on the long-term outcomes of neonatal jaundice.14,26 This is the only population dataset with different levels of bilirubin before phototherapy was widely used. These conditions make the CPP data particularly suitable for studying the exposure–effect relationships between neonatal bilirubin and childhood obesity. Finally, to the best of our knowledge, no studies have been published on neonatal bilirubin and childhood obesity. Additionally, our study has some limitations, and the main limitation of this study is that the CPP was completed 50 years ago. These preterm babies were born in the United States during the 1960s, and their birth weight, socioeconomic status, body composition, mothers’ age, mothers’ BMI before pregnancy, and mothers’ BMI gain during pregnancy may be different from those of preterm babies today. Many parts of the world are undergoing rapid economic development and social transition, and an increasing prevalence of obesity in children has been reported over the past several decades.2,3 However, the incidence of obesity in our study is similar to the prevalence estimates of obesity in preschool children (16%) from a contemporary study.27 Thus, our findings may have useful implications for infants in these areas. Second, in the CPP, the feeding methods data were collected only during the nursery stay, so we knew very little about the duration of breastfeeding, which has a significant dose–response effect on the reduced risk of childhood obesity.28 Some underlying diseases such as Gilbert’s syndrome, which may contribute to both neonatal jaundice and childhood obesity, were not recorded in our study due to its retrospective evaluation. In fact, socioeconomic status, caloric intake, and exercise levels during childhood are likely to be the majority of confounding and cannot be evaluated or adjusted for in this study. Another limitation may be the large amount of missing data: 1584 infants had no BMI values at age 7 years and were therefore excluded. We compared the TSB values between infants with and without BMI at age 7 years and found no significant differences (8.0 ± 4.9 vs. 8.1 ± 4.9, P = 0.46). For the same condition, BMI at age 7 years was also not significantly different between infants with and without missing CB values (15.8 ± 2.0 vs. 15.9 ± 2.1, P = 0.09). Thus, we have reasons to believe that the data are missing at random in our study.
References
World Cancer Research Fund. American Institute for Cancer Research Policy and Action for Cancer Prevention. Food, Nutrition, and Physical Activity: A Global Perspective (World Cancer Research Fund, 2009). http://www.dietandcancerreport.org/cancer_resource_center/downloads/chapters/pr/Introductory%20pages.
World Health Organization. Obesity and Overweight (World Health Organization, 2016). http://apps.who.int/mediacentre/factsheets/fs311/en/.
Lobstein, T., Baur, L. & Uauy, R. Obesity in children and young people: a crisis in public health. Obes. Rev. 5, 4–104 (2004).
Karadag, F. et al. Relationship between serum bilirubin levels and metabolic syndrome in patients with schizophrenia spectrum disorders. Clin. Psychopharmacol. Neurosci. 15, 153–162 (2017).
Cipolletta, D. et al. PPARγ is a major driver of the accumulation and phenotype of adipose-tissue Treg cells. Nature 486, 549–553 (2012).
Vítek, L. The role of bilirubin in diabetes, metabolic syndrome,and cardiovascular diseases. Front Pharmacol. 3, 55 (2012).
Choi, S. H., Yun, K. E. & Choi, H. J. Relationships between serumtotal bilirubin levels and metabolic syndrome in Koreanadults. Nutr. Metab. Cardiovasc. Dis. 23, 31–37 (2013).
Puri, K. et al. Serum bilirubin level is inversely associated with nonalcoholic steatohepatitis in children. J. Pediatr. Gastroenterol. Nutr. 57, 114–118 (2013).
Vera, T., Granger, J. P. & Stec, D. E. Inhibition of bilirubin metabolism induces moderate hyperbilirubinemia and attenuates ANG II-dependent hypertension in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R738–R743 (2009).
Bakrania, B. et al. Chronically elevated bilirubin protects from cardiac reperfusion injury in the male Gunn rat. Acta Physiol. (Oxf.). 220, 461–470 (2017).
Wallenstein, M. B. & Bhutani, V. K. Jaundice and kernicterus in the moderately preterm infant. Clin. Perinatol. 40, 679–688 (2013).
Tiribelli, C. & Ostrow, J. D. The molecular basis of bilirubin encephalopathy and toxicity: report of an EASL Single Topic Conference, Trieste, Italy, 1–2 October, 2004. J. Hepatol. 43, 156–166 (2005).
Gourley, G. R. Bilirubin metabolism and kernicterus. Adv. Pediatr. 44, 173–229 (1997).
Aspberg, S. et al. Is neonatal phototherapy associated with an increased risk for hospitalized childhood bronchial asthma? Pedia. Allergy Immunol. 18, 313–319 (2007).
Klebanoff, M. A. The Collaborative Perinatal Project: a 50-year retrospective. Paediatr. Perinat. Epidemiol. 23, 2–8 (2009).
Newman, T. B. & Klebanoff, M. A. Neonatal hyperbilirubinemia and long-term outcome: another look at the Collaborative Perinatal Project. Pediatrics 92, 651–657 (1993).
Vreman, H. J. et al. Interlaboratory variability of bilirubin measurements. Clin. Chem. 42, 869–873 (1996).
Kuczmarski, R. J. et al. CDC growth charts: United States. Adv. Data. 314, 1–27 (2000).
De Onis, M. et al. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 85, 660–667 (2007).
Jo, J. et al. Total, direct, and indirect serum bilirubin concentrations and metabolic syndrome among the Korean population. Endocrine 39, 182–189 (2011).
Amin, S. B. Clinical assessment of bilirubin-induced neurotoxicity in premature infants. Semin Perinatol. 28, 340–347 (2004).
Brown, C. et al. Family-based obesity treatment in children with disabilities. Acad. Pediatr. 15, 197–203 (2015).
Henson, M. B. et al. Food intake by brain-injured humans who are in the chronic phase of recovery. Brain Inj. 7, 169–178 (1993).
Shi, Y., De Groh, M. & Morrison, H. Perinatal and early childhood factors for overweight and obesity in young Canadian children. Can. J. Public Health 104, e69–e74 (2013).
Chi, D. L., Luu, M. & Chu, F. A scoping review of epidemiologic risk factors for pediatric obesity: Implications for future childhood obesity and dental caries prevention research. J. Public Health Dent. 77(Suppl. 1), S8–S31 (2017).
Huang, L. et al. Neonatal bilirubin levels and childhood asthma in the US Collaborative Perinatal Project, 1959–1965. Am. J. Epidemiol. 178, 1691–1697 (2013).
Twells, L. & Newhook, L. A. Can exclusive breastfeeding reduce the likelihood of childhood obesity in some regions of Canada? Can. J. Public Health 101, 36–39 (2010).
Yan, J. et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health 14, 1267 (2014).
Acknowledgements
This work was supported by the Sichuan Science and Technology Program [2019YJ0696] and the Southwest Medical University of China to X.L.
Author information
Authors and Affiliations
Contributions
L.L. and L.Z.: acquisition, analysis, and interpretation of data; drafting the article; W.D.: revising the article and final approval of the version to be published; Y.H. and H.Y.: acquisition of data; X.L.: substantial contributions to conception and design, revising the article, and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Luo, L., Zou, L., Dong, W. et al. Association between neonatal serum bilirubin and childhood obesity in preterm infants. Pediatr Res 86, 227–233 (2019). https://doi.org/10.1038/s41390-019-0399-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0399-3
This article is cited by
-
Associations between Neonatal Serum Bilirubin and Childhood Obesity in Term Infants
Scientific Reports (2019)