Abstract
Background
The aim was to clarify whether children born preterm with a history of necrotizing enterocolitis (NEC) had an increased risk of rickets, fractures, and/or vitamin D deficiency during childhood and adolescence compared to controls without NEC, matched for gestational age.
Methods
All infants born in Sweden between 1987 and 2009 with a gestational age <32 + 0 weeks and a diagnosis of NEC were identified. Totally, 465 children with a history of NEC and 2127 controls were included. International Classification of Diseases codes for all categories of fractures, rickets, vitamin D deficiency, and malnutrition were analyzed.
Results
In total, 94 of the 465 children with NEC died within 28 days. Of the 2127 controls, 288 died within 28 days. Among the remaining 371 NEC cases, 39 fracture occasions were identified. The 1839 controls had 204 fracture occasions. There was no significant difference in fractures. Rickets was diagnosed in 11 (3%) of the children with a history of NEC compared to 21 (1%) of the controls (odds ratio 2.65, 95% CI 1.26–5.53, p = 0.007).
Conclusions
This study showed an increased risk of rickets but not fractures during childhood and adolescence in children born preterm and with a history of NEC, compared to matched controls.
Similar content being viewed by others
Introduction
Necrotizing enterocolitis (NEC) is a severe inflammatory intestinal condition primarily affecting preterm infants. It is often associated with complications such as sepsis, perforation, peritonitis, and death.1 The overall incidence of NEC ranges from 0.34 to 1.8 per 1000 live births2,3,4,5 and is inversely proportional to birth weight and gestational age. Among the smallest and most preterm infants, the incidence of NEC can be over 10%.6,7,8 During the past decades, the number of surviving children born preterm has increased.9 Our group has previously presented national population-based studies on NEC epidemiology and trends in Sweden, which described an increase in the incidence of NEC between 1987 and 2009 and indications of space–time clustering on hospital level.2,10 The same cohort has now been used in the present study.
The pathogenesis of NEC is suggested to be multifactorial resulting from a combination of intestinal immaturity, abnormal microbial colonization, and immunoreactive intestinal mucosa.11,12 Severe cases of NEC are managed surgically with resection of the affected part of the intestine, leading to shortening of the bowel length, which may lead to malabsorption and intestinal failure.13,14,15,16 However, intestinal failure may also develop after medically treated NEC. In these cases, the intestinal failure is often resolving over time.15 Malabsorption of calcium and phosphorus may contribute to osteopenia and rickets following NEC in preterm infants.17
Osteopenia of prematurity, also known as metabolic bone disease of prematurity, remains an important cause of morbidity in preterm infants, especially among those with birth weights <1500 g.18,19,20,21,22 Risk factors for osteopenia of prematurity include intestinal surgery, extreme preterm birth, extreme low birth weight, maternal preeclampsia, and delayed enteral feeding. Glucocorticoids and diuretics, which are commonly used in preterm infants, also increase the risk.23,24
Children’s bone health is of increasing importance and concern. Sweden has among the highest incidence of osteoporosis and fractures in the world.25 Under normal conditions, bone mass increases steadily and reaches a plateau in early adulthood, with the bone mass achieved serving as a “bone bank” for the remainder of life. Some studies indicate that high peak bone mass reduces the risk of osteoporotic fractures later in life. It is thus important to maximize the acquisition of bone mass during childhood.26 Factors such as genetics, physical activity, hormones and vitamins like vitamin D, nutrition, as well as chronic disorders and medications, influence bone mineral accrual.21
Rickets of prematurity is mainly attributable to calcium and phosphorus deficiencies, in contrast to nutritional rickets in childhood that is usually caused by vitamin D deficiency.27,28 In infants with cholestasis, however, vitamin D deficiency is described following malabsorption of fat-soluble vitamins.29 Maximum fetal accretion rates for calcium and phosphorus occur in the third trimester, when 80% of these minerals in the fetal skeleton are retained. Consequently, infants born preterm have low supplies of these minerals.30 Symptoms of rickets in infancy can be, e.g., thickened wrists, enlarged size of the fontanelle, deformity, and delayed walking and, in older children, bowed legs.31 Preterm infants have been shown to have low bone mass at discharge from hospital with a possible catch-up during infancy and childhood.32,33,34 NEC with malabsorption of calcium and phosphorus may aggravate the metabolic bone disease of prematurity. Therefore, we hypothesized that children with a history of NEC would have an increased risk for fractures and rickets compared to controls. To the best of our knowledge, this has not been previously studied longitudinally.
The aim of this study was to clarify whether children born preterm and diagnosed with NEC had an increased risk of rickets, fractures, and/or vitamin D deficiency during childhood and adolescence compared to control individuals, matched for gestational age but without a history of NEC.
Methods
Subjects
A cohort of all infants with a diagnosis of NEC born in Sweden between 1987 and 2009 was identified from registers held by the Swedish National Board of Health and Welfare: the National Patient Register (NPR), the Swedish Medical Birth Register (SMB), and the National Cause of Death Register (NCD). The NPR covers all hospital admissions and specialist outpatient care in Sweden. The diagnose codes of NEC according to the Ninth or Tenth revisions of the International Classification of Diseases (ICD) were used: ICD-9 code 777F and ICD-10 code P77. Between 1987 and 1996, the Ninth revision of ICD was used, and from 1997 the Tenth revision of ICD was used.
Because of the rarity of NEC and the concentration of NEC to the premature children, we used a case-control design with matching on gestational age and birth year. Further detail of design of the study and the process of data collection has been described in detail in our previous publications.2,10
A total of 720 children with a history of NEC and complete information in the SMB were identified during the years 1987–2009. Controls were selected randomly, matched for gestational age and birth year. For a majority of the NEC cases, six controls were found. However, in the most preterm subgroups, some NEC cases had fewer controls due to a limited number of eligible controls. In total, 3656 controls were identified. This imbalance of controls should not induce any bias.
In term infants, NEC is often related to an underlying condition such as cardiac malformation or asphyxia. This study focused on preterm infants with NEC, and only infants with a gestational age <31 + 6 were included. The study population thus consisted of 465 NEC cases and 2127 controls.
Only the infants surviving >28 days were eligible for analyses of morbidities related to metabolic bone disease of prematurity. Infants surviving <28 days were excluded from further analysis.
Variables
The outcomes for the cases and controls were assessed up to December 31, 2012 through linkage with the NPR. The NPR contains discharge diagnoses according to the ICD coding system for all hospital admissions in Sweden. Diagnoses analyzed in this study were all categories of fractures (Table 2), rickets, vitamin D deficiency, short stature, disturbances in physiological development, malnutrition, malabsorption, and obesity (Table 4). Moreover, diagnostic codes for osteoporosis (M80-82), osteomalacia (M83), disorders of bone mineral density and structure (M85), disorders of bone development and growth (M89), and deficient production of growth hormone/hypofunction of pituitary gland (E23.0A) were studied.
The occurrence of each diagnostic code was compared between NEC cases and controls.
The 465 NEC cases included both medically and surgically treated NEC. Surgical NEC cases were identified using the 1988, 1996, and 2004 versions of the Swedish Classification of surgical procedures.
Protocol and statistical analysis
Each diagnosis, except for fractures, was only counted once per individual, even if it generated multiple admissions as recorded in the NPR. As for fractures, the reoccurrence of the same code within 1 year was counted as one episode. If the code was used at a later occasion, it was counted as a new fracture episode. Hence, a fracture code could be retrieved several times from one individual, indicating more than one fracture during childhood and adolescence. It was also noted how many unique individuals that had fractures. Both the number of fractures and the number of individuals with fractures were taken into consideration.
Differences in the variables were analyzed using Chi-square test and Fischer’s exact test for categorical data and Mann–Whitney U test for quantitative data. Because of the different length of follow-up, the comparisons in risk for fractures were made with Kaplan–Meier estimates and univariate and multivariate Cox proportional hazards regression with December 31, 2012 as latest date of follow-up. The significance level was set at p < 0.05. SPSS statistics version 23 and Stata release 1535 were used to conduct all analyses.
The study was approved by the Regional Ethical Review Board in Linköping, approval number M213-07 with supplement 2010/405-32.
Results
Patient characteristics
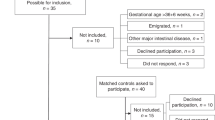
The characteristics of the study population are shown in Table 1. Regarding gender, 55% of the study population was boys, without significant difference between NEC cases and controls. There was a significant difference between the two groups in gestational age, explained by the unequal distribution of controls with fewer controls per case in the most preterm children. A significant difference was also seen in birth weight, where NEC patients and controls had a mean birth weight of 995 and 1126 g, respectively. A total of 114 (24.5%) children with NEC and 5 (0.2%) children in the control group were from multiple pregnancies, p < 0.001. No statistical difference was noted between the groups regarding being born small for gestational age, defined as being born below −2 standard deviations of expected birth weight in relation to gestational age. A higher proportion of infants died within 7 days of life in the control group (11.5%) compared to NEC cases (6.7%), p = 0.002, but the mortality within 28 days was higher in the NEC group, 20.2% versus 13.5% in the control group, p < 0.001. In total, 94 of the 465 children with NEC died within 28 days, leaving 371 children for analyses of diagnostic codes regarding long-term morbidities related to metabolic bone disease. Of the 2127 controls, 288 died within 28 days, leaving 1839 individuals for morbidity analyses. The study identified 120 surgical NEC cases, of which 112 survived >28 days (Fig. 1).
Fractures
Of the remaining 371 NEC cases, 39 fracture occasions in 29 individuals were identified (Table 2). One child had a fracture at the same location three times, one child had a fracture at the same location two times, three of the children had fracture diagnoses at two different locations, and two children had fracture diagnoses at three different locations. Of the remaining 1839 controls, 204 occasions of fractures in 164 individuals were identified (Table 2). Two of the children had a fracture at the same location 3 times, 9 children had a fracture at the same location 2 times, 16 children had fracture diagnoses at 2 different locations, 4 children had fracture diagnoses at 3 different locations, and 1 child had fractures at 4 different locations. Of the 29 NEC cases with fractures, 19 were boys. Of the 164 controls with fractures, 100 were boys. Statistical analyses were made on the total number of fractures and for every subgroup of fractures. As shown in Table 2, the incidence of fractures did not differ significantly between NEC cases and controls, neither the total number of fractures nor in any of the subgroups. NEC cases were significantly older than controls at the time of fracture with a mean age at time of fracture of 11.2 years in the NEC group and 9.2 years among controls (p = 0.037).
According to Kaplan–Meier estimate and Cox regression analyses, the NEC cases had similar risk for fractures as the controls (Fig. 2 and Table 3). Girls had a significant lower risk compared with boys. Children with >27 weeks of gestational age had a higher risk compared with children with lower gestational age. When analyzing fractures among the surgical NEC cases compared to controls, no differences could be found (data not shown).
Kaplan–Meier estimate showing the risk of fractures in relation to the number of years studied. Data were collected from children born between 1987 and 2012. The children had different ages at the time of data collection. No difference regarding the risk of fractures could be detected between necrotizing enterocolitis cases and controls
Rickets
Eleven (3%) of the children with NEC were diagnosed with rickets compared to 21 (1.1%) of the controls (odds ratio (OR) 2.65, 95% confidence interval (CI) 1.26–5.53, p = 0.007) (Table 4). The diagnosis of rickets was made during the first year of life in all NEC patients and in all but one of the controls. One control was diagnosed at 13 years of age.
Of the patients diagnosed with rickets, only two had any fracture diagnosis, both from the control group. One of them was the child diagnosed with rickets at age 13 years. This patient had four different fracture occasions, including a scapula fracture.
Vitamin D deficiency and other related diseases
No child, neither in the NEC group nor in the control group, was diagnosed with vitamin D deficiency.
Intestinal malabsorption, postsurgical malabsorption, and postprocedural disorder of the digestive system were significantly more common in the NEC group (Table 4).
Short stature, malnutrition, retarded development following malnutrition, other nutritional deficiencies, sequelae of malnutrition, lack of expected normal physiological development including failure to thrive, and obesity did not differ significantly (Table 4).
Discussion
This study showed an increased risk of rickets during infancy in children with a history of preterm birth and NEC compared to preterm children without NEC. However, a history of NEC was not associated with an increased risk of fractures during childhood and adolescence. Notable is that children with >27 weeks of gestational age had a higher risk compared with children with lower gestational age. The reason for this or any clinical implication is not possible to assess from our data. Furthermore, the diagnosis malabsorption was more common among NEC cases than controls. Only the infants surviving >28 days were eligible for analyses of morbidities related to metabolic bone disease of prematurity. Mortality within 28 days was higher in the NEC group, but 7-day mortality was higher among controls. This is explained by the fact that NEC commonly develops after 7 days of life, and the most severely ill infants would die before developing NEC.
Osteopenia of prematurity is a well-known and important cause of morbidity in preterm infants. It was previously shown that NEC in preterm infants is associated with an increased risk of developing osteopenia of prematurity.18,19,36 Nutritional rickets following NEC was described already in 1986 by Cikrit et al.37
In the present study, we could confirm these findings with an increased proportion of children with rickets in children with a history of preterm birth and NEC compared to preterm children without NEC. The study was limited to the diagnostic codes from ICD 9 and ICD 10 and could not confirm the diagnosis by X-ray or by following laboratory results, such as alkaline phosphatase or s-phosphate. The overall incidence of rickets was low with 3.0% in the NEC group and 1.1% among the controls. Whether metabolic bone disease of prematurity is a condition mainly limited to the neonatal period is not fully studied. As previously mentioned, preterm infants in general have low bone mass at discharge from hospital with a possible catch-up during infancy and childhood.32,33,34 No child in the NEC group and only one child in the control group was diagnosed with rickets after 1 year of age. Our group has earlier presented results showing that children born preterm have normal bone mass but different body composition compared to full-term controls at 4 years of age.36
In modern neonatology with increasing awareness of the risk factors for metabolic bone disease and a focus on improved nutrition, the aim is to further limit the number of cases with neonatal osteopenia and rickets. A recent survey among members of the American Academy of Pediatrics shows that there is a good awareness of metabolic bone disease of prematurity among neonatologists in the USA, but there is still a need to optimize strategies to prevent and manage the condition.38
NEC is a risk factor for metabolic bone disease of prematurity but the long-term effects after the neonatal period are not sufficiently studied. No differences in the number of or type of fractures during childhood and adolescence were found between children with a history of preterm birth and NEC compared to matched controls. It was a reassuring to find that NEC during the neonatal period among preterm infants does not seem to increase the risk of fractures during childhood. We have no explanation for the significantly older age at the time of fracture in NEC patients compared to controls. Any differences in daily life activities between the groups cannot be discerned from our data.
Among preterm infants with a history of NEC, there are several factors that might contribute to osteopenia. NEC, both medically and surgically treated, may lead to a dysfunctional bowel with malabsorption of calcium and phosphorus. In this study, diagnoses related to malabsorption were more common in the NEC group than in controls. Among infants with intestinal failure treated with parenteral nutrition for a longer period, cholestasis may develop leading to malabsorption of fat-soluble vitamins.29,39 This may lead to vitamin D deficiency, which could aggravate the metabolic bone disease. In the present study, however, there was not a single child diagnosed with vitamin D deficiency according to the reported diagnose codes. Owing to the retrospective design of this study, it was not possible to analyze the level of 25-OH vitamin D. In Sweden, vitamin D supplementation is recommended to all children until the age of 2 years.
Strength and weaknesses
A strength of the present study is the cohort of all newborn infants with a diagnosis of NEC born in Sweden between 1987 and 2009 resulting in a large number of studied patients and controls followed for a long period of time.
A weakness is the retrospective design, which makes it impossible to follow-up laboratory and clinical data to confirm the diagnosis. Furthermore, all diagnostic data is based on the diagnostic codes as they are reported by the treating clinician. The use of patient registers in this study not only includes the advantage of being able to study large number of patients but also includes the disadvantage of not being able to confirm the diagnosis on each patient with a medical record review.
Conclusion
In summary, this longitudinal follow-up study showed that, among children born preterm, those with a history of NEC were at increased risk of rickets and diagnosis of malabsorption than those without NEC. However, a history of NEC was not associated with an increased risk of fractures during childhood and adolescence. The diagnosis of rickets was usually limited to the first year of life. Children with a history of NEC were older, compared to controls, at the time of fractures. Children born between gestational weeks 28–32 had a higher risk of fractures compared to extremely preterm infants.
References
Rojas, S. et al. Abdominal pathology requiring laparotomy in very preterm infants is associated with need for surgical closure of patent ductus arteriosus. J. Pediatr. Surg. 46, 1898–1902 (2011).
Ahle, M., Drott, P. & Andersson, R. E. Epidemiology and trends of necrotizing enterocolitis in Sweden: 1987-2009. Pediatrics 132, e443–e451 (2013).
Papillon, S. et al. Necrotizing enterocolitis: contemporary management and outcomes. Adv. Pediatr. 60, 263–279 (2013).
Niemarkt, H. J. et al. Necrotizing enterocolitis: a clinical review on diagnostic biomarkers and the role of the intestinal microbiota. Inflamm. Bowel Dis. 21, 436–444 (2015).
Lim, J. C., Golden, J. M. & Ford, H. R. Pathogenesis of neonatal necrotizing enterocolitis. Pediatr. Surg. Int. 31, 509–518 (2015).
Stoll, B. J. et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126, 443–456 (2010).
Yee, W. H. et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 129, e298–e304 (2012).
Guillet, R. et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 117, e137–e142 (2006).
Fellman, V. et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 301, 2225–2233 (2009).
Magnusson, A. et al. Population-based study showed that necrotising enterocolitis occurred in space-time clusters with a decreasing secular trend in Sweden. Acta Paediatr. 106, 1097–1102 (2017).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. New Engl. J. Med. 364, 255–264 (2011).
Eaton, S., Rees, C. M. & Hall, N. J. Current research in necrotizing enterocolitis. Early Hum. Dev. 97, 33–39 (2016).
Berman, L. & Moss, R. L. Necrotizing enterocolitis: an update. Semin. Fetal Neonatal Med. 16, 145–150 (2011).
Sharma, R. & Hudak, M. L. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin. Perinatol. 40, 27–51 (2013).
Pironi, L. Definitions of intestinal failure and the short bowel syndrome. Best. Pract. Res. Clin. Gastroenterol. 30, 173–185 (2016).
Vanderhoof, J. A. & Langnas, A. N. Short-bowel syndrome in children and adults. Gastroenterology 113, 1767–1778 (1997).
Cakir, M. et al. Necrotizing enterocolitis increases the bone resorption in premature infants. Early Hum. Dev. 82, 405–409 (2006).
Harrison, C. M. & Gibson, A. T. Osteopenia in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 98, F272–F275 (2013).
Harrison, C. M., Johnson, K. & McKechnie, E. Osteopenia of prematurity: a national survey and review of practice. Acta Paediatr. 97, 407–413 (2008).
Dabezies, E. J. & Warren, P. D. Fractures in very low birth weight infants with rickets. Clin. Orthop. Relat. Res. 335, 233–239 (1997).
Abrams, S. A. Calcium and vitamin D requirements of enterally fed preterm infants. Pediatrics 131, e1676–e1683 (2013).
Carpenter, T. O. et al. Rickets. Nat. Rev. Dis. Prim. 3, 17101 (2017).
Chin, L. K. et al. Outcomes of standardised approach to metabolic bone disease of prematurity. J. Paediatr. Child Health 54, 665–670 (2018).
Dokos, C. et al. Inside the “fragile” infant: pathophysiology, molecular background, risk factors and investigation of neonatal osteopenia. Clin. Cases Miner. Bone Metab. 10, 86–90 (2013).
Kanis, J. A. et al. International variations in hip fracture probabilities: implications for risk assessment. J. Bone Min. Res. 17, 1237–1244 (2002).
Heaney, R. P. et al. Peak bone mass. Osteoporos. Int. 11, 985–1009 (2000).
Tsai, J. R. & Yang, P. H. Rickets of premature infants induced by calcium deficiency. A case report. Chang. Yi Xue Za Zhi 20, 142–147 (1997).
Mimouni, F. B. et al. Calcium, phosphorus, magnesium and vitamin D requirements of the preterm infant. World Rev. Nutr. Diet. 110, 140–151 (2014).
Jensen, M. et al. Difficulty achieving vitamin D sufficiency with high-dose oral repletion therapy in infants with cholestasis. J. Pediatr. Gastroenterol. Nutr. 61, 187–189 (2015).
Rohana, J., Hasmawati, J. & Zulkifli, S. Z. Risk factors associated with low bone mineral content in very low birth weight infants. Singap. Med J. 48, 191–194 (2007).
Wharton, B. & Bishop, N. Rickets. Lancet 362, 1389–1400 (2003).
Congdon, P. J. et al. Spontaneous resolution of bone mineral depletion in preterm infants. Arch. Dis. Child. 65(10 Spec No), 1038–1042 (1990).
Pittard, W. B. et al. Longitudinal changes in the bone mineral content of term and premature infants. Am. J. Dis. Child. 144, 36–40 (1990).
Rubinacci, A. et al. Is there an impact of birth weight and early life nutrition on bone mineral content in preterm born infants and children? Acta Paediatr. 82, 711–713 (1993).
StataCorp. Stata Statistical Software: Release 15 (StataCorp LLC, College Station, TX, 2017).
Stigson, L. et al. Bone and fat mass in relation to postnatal levels of insulin-like growth factors in prematurely born children at 4y of age. Pediatr. Res. 75, 544–550 (2014).
Cikrit, D. et al. Long-term follow-up after surgical management of necrotizing enterocolitis: sixty-three cases. J. Pediatr. Surg. 21, 533–535 (1986).
Kelly, A., Kovatch, K. J. & Garber, S. J. Metabolic bone disease screening practices among U.S. neonatologists. Clin. Pediatr. (Philos.) 53, 1077–1083 (2014).
Elfvin, A. et al. Low birthweight, gestational age, need for surgical intervention and gram-negative bacteraemia predict intestinal failure following necrotising enterocolitis. Acta Paediatr. 104, 771–776 (2015).
Acknowledgements
This study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (797211, 727721, 716831 and 678871), and financial support by research grants from the Gothenburg medical society and from the Queen Silvia Children’s hospital research foundation.
Author information
Authors and Affiliations
Contributions
A.M.: Conception and design of the study, acquisition of data and analysis and interpretation of data, drafting the article and revising it critically, and final approval of the version to be published. M.A. and R.E.A.: Conception and design of the study, acquisition of data and analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be published. D.S.-E.: Conception and design of the study, analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be published. A.E.: Conception and design of the study, analysis and interpretation of data, drafting the article and revising it critically, and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Magnusson, A., Ahle, M., Andersson, R.E. et al. Increased risk of rickets but not fractures during childhood and adolescence following necrotizing enterocolitis among children born preterm in Sweden. Pediatr Res 86, 100–106 (2019). https://doi.org/10.1038/s41390-019-0390-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0390-z
This article is cited by
-
Body composition and bone mass among 5-year-old survivors of necrotizing enterocolitis
Pediatric Research (2023)
-
Establishment of a nomogram model for predicting metabolic bone disease in preterm infants: A case‒control study
European Journal of Pediatrics (2023)