Abstract
Study objectives
Current evidence in adults suggests that, independent of obesity, obstructive sleep apnea (OSA) can lead to autonomic dysfunction and impaired glucose metabolism, but these relationships are less clear in children. The purpose of this study was to investigate the associations among OSA, glucose metabolism, and daytime autonomic function in obese pediatric subjects.
Methods
Twenty-three obese boys participated in: overnight polysomnography; a frequently sampled intravenous glucose tolerance test; and recordings of spontaneous cardiorespiratory data in both the supine (baseline) and standing (sympathetic stimulus) postures.
Results
Baseline systolic blood pressure and reactivity of low-frequency heart rate variability to postural stress correlated with insulin resistance, increased fasting glucose, and reduced beta-cell function, but not OSA severity. Baroreflex sensitivity reactivity was reduced with sleep fragmentation, but only for subjects with low insulin sensitivity and/or low first-phase insulin response to glucose.
Conclusions
These findings suggest that vascular sympathetic activity impairment is more strongly affected by metabolic dysfunction than by OSA severity, while blunted vagal autonomic function associated with sleep fragmentation in OSA is enhanced when metabolic dysfunction is also present.
Similar content being viewed by others
Introduction
Obesity is considered to be a risk factor for hypertension and Type 2 diabetes, both of which predispose to cardiovascular disease.1 As well, obesity is a major risk factor for the development and progression of obstructive sleep apnea (OSA).2 A growing body of evidence suggests that OSA itself can contribute independently to the development of systemic hypertension, coronary artery disease, heart failure, and stroke.3,4 More recently, many studies have pointed to an association between OSA and insulin resistance (IR), independent of obesity.5,6 One factor that is common to obesity, OSA and IR is sympathetic nervous system overactivity.7 It remains unclear as to how these factors are causally connected, but it is likely that the vicious cycle of interplay among these factors predisposes to the emergence of “metabolic syndrome”, a convergence of obesity, hypertension, IR, and dyslipidemia.8 This same constellation of factors is becoming more prevalent in children,9 increasing the risk for cardiovascular disease in the long term. In this regard, studies on obese children may provide better insights into the development of metabolic and autonomic dysfunction in OSA, due to the fact that children are less likely than adults, by virtue of age, to host co-morbidities that could confound measurements of cardiometabolic status. Nevertheless, to date, no comprehensive studies have yet been performed on the pediatric population to examine these associations in a single study, with the same patients.
The study hypothesis was that OSA indices and metabolic status are related to autonomic disorders, measured by heart rate variability and baroreflex sensitivity. In contradistinction to a number of similar studies that exist in the literature, our work investigated the associations among the three major factors in the same patients, who participated in a single study. Although prospective, longitudinal studies will ultimately be needed to definitively establish causal links, we postulated that careful quantitative analysis of the relationships linking measures of autonomic and metabolic function to OSA severity in the same pool of subjects would provide a useful foundation for future mechanistic studies.
Methods
Subjects
Twenty-three obese Latino male adolescents were recruited over a period of 24 months from the pool of patients referred to the Children’s Hospital Los Angeles (CHLA) Pulmonary Division for evaluation of snoring. Participants were required to be overweight or obese based on BMI Z-score and were otherwise healthy. Adolescence was defined as older than 10 years. All subjects were recruited either after referral for polysomnography or to the pulmonary sleep clinic for a complaint of snoring with suspicion OSA. Exclusion criteria included a history of lung disease other than mild asthma not requiring inhaled corticosteroids, diabetes mellitus, hypertension requiring the use of antihypertensive medications, use of stimulant medications, and positive airway treatment for OSA. Subjects completed an overnight polysomnography (PSG), a frequently sampled intravenous glucose tolerance test (FSIVGTT), a dual-energy X-ray absorptiometry (DEXA) for assessing adiposity, and noninvasive measurement of respiration, heart rate (HR), and continuous blood pressure (BP) during the supine and standing postures. Only subjects that completed all components of the study were considered in the present investigation. A detailed description of the measurements of body fat, indexes of metabolic function, and polysomnography can be found in a previous paper.10
Latino youth are at increased risk for the development of cardiovascular disease, type 2 diabetes mellitus, and other complications of the metabolic syndrome.11 Studies investigating this population are limited.12 Moreover, since insulin resistance and compensatory insulin secretion vary with ethnicity and gender,12,13 the current study was limited to obese adolescent Latino males to reduce the number of confounding variables. Furthermore, while the FSIVGTT allows a superior assessment of glucose homeostasis when compared to fasting values alone, the invasive nature of this test precluded the recruitment of healthy control subjects.
The experimental protocol was approved by the CHLA Institutional Review Board. Assent was obtained from each subject and written informed consent from the subject’s parents before participation in the study.
Indices of OSA severity
Overnight sleep parameters considered included the obstructive apnea hypopnea index (OAHI), the desaturation index (DESAT), and the total arousal index (TAI), per AASM scoring recommendations.14 The latter (TAI) includes both spontaneous and respiratory related arousals.
The AASM scoring recommendations show that there is still no consensus on the respiratory scoring rules to use for children between 13 and 18 years of age. In children, mild OSA is characterized by an OAHI between 1.5 and 5 events/h, moderate OSA between 5 and 10 events/hour, while severe OSA is characterized by OAHI greater than 10 events/hour.15,16 Based on these criteria, most of the subjects had mild apnea (16 cases, or 69.6%), 6 (26.1%) had moderate to severe apnea, while 1 subject (4.3%) had primary snoring with an OAHI less than 1.5 events/hour. Since exclusion criteria included co-morbidities such as asthma, diabetes, and systemic hypertension, potentially children with severe OSA were also excluded from the study. To divide our study population into “low” and “high” OAHI, we used the average of our study population (3 events/hour) as cutoff for OAHI. Using 5 events/h as cutoff did not significantly change our results.
Indices of metabolic function
To characterize the metabolic function of subjects, the following indices based on blood sampling obtained after overnight fasting were determined: (i) fasting plasma glucose concentration; (ii) insulin concentration levels; (iii) Homeostasis Model Assessment of Insulin Resistance index, or HOMAIR index, based on fasting blood glucose and insulin levels.17,18 High HOMAIR scores denote high insulin resistance (or, equivalently, low insulin sensitivity). To characterize the dynamics of glucose metabolism in the presence of insulin, frequently sampled intravenous glucose tolerance test (FSIVGTT) was employed, yielding accurate estimates of IR and also information about beta-cell function.19,20 The protocol adopted in this study was the insulin-modified FSIVGTT, consisting of timed blood samples after an overnight fast (12 h). Glucose and insulin levels measured during the FSIVGTT were analyzed by a mathematical model of glucose homeostasis by insulin.21
The metabolic parameters used to determine insulin-glucose physiology considered in this study, estimated from the FSIVGTT measurements, were: insulin sensitivity, SI ( × 10−4ml/min/μU), acute insulin response to glucose, AIRg (μU/ml × min), disposition index, DI ( × 10−4 /min), and glucose effectiveness, SG (min−1).
Indices of autonomic function
Indices of autonomic function were obtained for each subject in the daytime, consisting of contiguous 10-min periods with measurements of airflow, continuous BP, and HR (ECG), initially in the supine (baseline) posture and, subsequently, while standing (orthostatic challenge as a sympathetic stimulus).
All patients were instructed to maintain their normal breathing pattern throughout the study. Airflow was measured via a pneumotachometer (model 3700, Hans Rudolph, Kansas City, MO) connected to a snug-fitting mask that covered the subjects’ nose and mouth. The variation of the lung volume over time was obtained from integration of airflow measured using the pneumotachograph connected to a differential pressure transducer (Validyne Model MP45-1-871 variable-reluctance transducer, from Validyne Engineering Corp., Northridge, CA). HR was monitored with a standard three-lead configuration and amplified using a BMA-200 bioamplifier, CWE Inc, Ardmore, PA. Systolic, mean, and diastolic BP signals were obtained from the continuous BP waveform recorded using a Nexfin HD monitor (BMEYE B. V., Amsterdam, the Netherlands).
With the subject in the supine posture for at least 5 min, the subject’s cuff BP was initially measured and the mean of three measurements taken at 1 min intervals is used as reference. The mean of the BP measurements obtained from the Nexfin device during supine was adjusted to match the reference average cuff BP for each subject. Subsequently, 10 min of data were recorded for determining baseline autonomic activity. The same variables were then recorded (10 min) with the subject in the standing posture, in order to elicit a shift of sympathovagal balance towards relative sympathetic dominance.22 Recording of the standing autonomic measurements began after the subject had assumed the upright posture for 5 min for hemodynamic equilibration.
Indices of the autonomic function determined from these measurements, for each posture, included: (a) mean R-R interval (mRRI); (b) mean systolic and diastolic BP (mSBP and mDBP, respectively); (c) low and high-frequency power of RRI variability (RRILF and RRIHF, respectively); (d) the ratio of the low- to high-frequency power of RRI variability (RRILHR); (e) low and high-frequency power of BP variability (SBPLF and SBPHF, respectively); (f) the baroreflex sensitivity (BRS) index, both in the low and high-frequency ranges (BRSLF and BRSHF, respectively).
BRS is an index of arterial baroreflex function.23 More specifically, it is a measure of a change in HR (or, more precisely in this study, its reciprocal, the R-to-R interval, RRI) per unit change in BP (systolic BP, SBP), in ms/mmHg. The higher the BRS index, the stronger the system’s response to a change in BP.23 In the present study, BRS was calculated as the square root of the ratio of the area under the curve of the RRI power spectrum by the area under the curve of the SBP power spectrum, both in the LF and HF regions:24
Supine (baseline) vs. standing autonomic descriptors
Postural change from supine to standing was used as an autonomic challenge to elicit a shift in cardiac autonomic modulation from parasympathetic (supine) to sympathetic dominance (standing).22 The autonomic HR variability (HRV), BP variability (BPV), and BRS indices were obtained from data collected in both the supine and standing postures. To quantify the autonomic reactivity to postural change, we used an autonomic reactivity index rRRI, defined as the ratio of the standing divided by the supine values, calculated for both low and high-frequency components (rRRILF and rRRIHF, respectively), as well as for median RRI (rmRRI). A stand/supine ratio that is close to unity would represent low autonomic reactivity to orthostatic stress, whereas a ratio either much higher or much lower than unity would suggest a high autonomic reactivity. Similarly, a baroreflex reactivity index, rBRS, was used to quantify the BRS reactivity to postural change, in both the low and high-frequency regions (rBRSLF and rBRSHF, respectively).
Statistical analyses
The study hypothesis was that OSA indices and metabolic status are related to autonomic disorders, measured by HR and BP variabilities and BRS indices calculated in the frequency domain. The variables considered in this study are listed in Table 1. All variables were tested for normality (Kolmogorov–Smirnov test). Variables that were not normally distributed were log-transformed before analysis.
Analysis of variance
Initially, one-way analysis of variance was used to test for difference in autonomic status, measured by the cardiovascular metrics listed in Table 2, between different metabolic or sleep groups. The metabolic and sleep variables considered are listed in Table 1. For this test, all independent variables were dichotomized at or near their medians, in order to divide the subjects into “low” and “high” sub-groups of roughly the same size. For example, our median cutoff for SI and DI are 2.3 and 2200, respectively, which is in accordance to the mean values for SI and DI found in Goran et al.25 at baseline (2.31 and 2583, respectively).
Subsequently, two-way ANOVA tests with interaction were performed on each of the autonomic indices (SBP, DBP, mRRI, RRILF, RRIHF, RRILHR, SBPLF, SBPHF, or BRSLF, BRSHF), in each posture (supine and standing, as well as the ratio stand/supine), with SDB (OAHI, TAI, or DESAT) and metabolic (SI, AIRg, DI, SG, fasting glucose, fasting insulin, or HOMAIR) status as the independent variables.
Multiple linear regression
The relationships between each of the autonomic indices and the potential explanatory variables (OSA and metabolic parameters), considering the influence of age and adiposity, were further investigated using multiple linear regression (MLR), with the following independent variables:
-
(a)
model A: OSA parameter, age, total % body fat
-
(b)
model B: metabolic index, age, total % body fat
-
(c)
model C: OSA parameter, metabolic index, age, total % body fat
For each model, the standardized regression coefficient βrel and its corresponding p-value, as well as the r2 statistic, were determined. The standardized regression coefficient βrel, with standardized units, quantifies the relative strength of each explanatory variable on the outcome (the autonomic measure considered).26
Finally, motivated by the fact that many two-way ANOVA with interaction tests showed a significant interaction term, a multiple regression analysis with interaction was performed, with centered predictors to avoid spurious regression effects.27
Results
The characteristics of the subjects in all studies performed are displayed in Table 1.
HRV indices—spectral analysis
There were no significant associations between mean RRI or the HRV indices with supine or standing postures and either metabolic or OSA parameters. As well, no baseline autonomic index obtained from spectral analysis of HRV was found to be significantly associated with any sleep or metabolic measures, by any of the statistical tests evaluated (1-way and 2-way ANOVA, multiple regression analyses).
However, RRILHR increased significantly from supine to standing (p < 0.001) in all subjects, suggesting a shift in autonomic balance towards sympathetic dominance. Both mRRI and RRIHF for all subjects decreased in the standing relative to supine (p < 0.0001 in both cases, paired t-test, as shown in Table 2), implying decreased vagal modulation following orthostatic stress. The ratio rRRIHF was less than 1 for all subjects, indicating a decrease in vagal modulation upon standing, as expected. The ratio rmRRI was also <1 for all subjects, indicating increased median HR in the standing posture compared to baseline. The shorter R-R intervals (or higher HR) in the standing posture are consistent with a decrease in parasympathetic modulation.22
Standard MLR analysis found the log-transformed rRRILF ratio to be positively correlated with log(SI), but not with any of the OSA parameters. Figure 1 shows the plot for the regression of SI on the ratio rRRILF, adjusted for age and adiposity. Subjects with higher SI values (above 5) have mostly an rRRILF ratio > 1 while those with low SI values (<5) predominantly have a rRRILF ratio < 1.
BP and BPV
DBP increased significantly from supine to standing, while SBP remained unchanged, consistent with what has been previously reported.28 In terms of the BPV indices, both low and high-frequency components (SBPLF and SBPHF, respectively) increased from supine to standing. The increase in SBPLF could be an indication of increased sympathetic modulation of peripheral vasculature, whereas the increase in SBPHF may have been due to enhanced respiratory modulation of the contractility of the heart29,30 during standing.
Comparing SBPsup (baseline) between different metabolic or sleep groups, SBPsup was higher in subjects with insulin resistance, defined by low SI and elevated fasting glucose and HOMAIR values (all p < 0.02, one-way ANOVA). However, SBPsup was not affected by OSA parameters, such as OAHI, TAI or DESAT (p > 0.05 for all comparisons, one-way ANOVA). Two-way ANOVA and MLR analyses, including both metabolic and OSA indices as independent variables confirmed these results. Table 3 presents the results of the significant MLR analyses, including the standardized regression coefficient βrel and its corresponding p-value, for SBPsup as the dependent variable. Figure 2 shows the scatter plot and regression line of SBPsup vs. SI, while Fig. 3 shows the results for SBPsup vs. fasting glucose, both adjusted for age and total % body fat. The MLR tests showed an increase in SBPsup with lower disposition index (DI) levels, but not with any of the sleep parameters analyzed. There was no significant association between any of the OSA severity indices or any of the metabolic indices and the low and high-frequency components of BPV (SBPLF and SBPHF, respectively) or the BP reactivity indices (stand-to-supine ratios, in either frequency range).
Baroreflex sensitivity (BRS)
No association was found in BRSLF and BRSHF (in both supine and standing) among different metabolic or sleep groups (using either ANOVA or regression analyses). However, both BRSLF and BRSHF decreased from supine to standing.
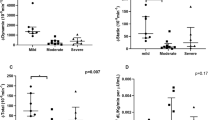
The reactivity index, rBRSHF, increased with sleep fragmentation, as measured by TAI, indicating a reduced baroreflex reactivity to postural stress with increased TAI (p < 0.01 for both one- and two-way ANOVA), while no main effect was observed for any metabolic index (p > 0.05, both one- and two-way ANOVA). Nevertheless, a post hoc analysis using Tukey’s procedure further revealed that, for those subjects with low SI, a higher sleep fragmentation (as measured by TAI) is associated with a decreased BRSHF reactivity to postural change. No difference in rBRSHF was found in the high SI group. Since all subjects showed decreases in BRSHF and BRSLF with postural change, indicating a decreased baroreflex sensitivity in the standing posture, all rBRS stand-to-supine ratios were < 1. However, a higher ratio (an rBRS value closer to 1) indicates a smaller degree of change from supine to standing, suggesting a decreased BRS reactivity to postural change. This result is illustrated in Fig. 4a.
Similarly, while no main effect was observed for AIRg (p = 0.17, two-way ANOVA), a post hoc pairwise multiple comparison using Tukey’s procedure further revealed that, within the low AIRg group, an increase in TAI was associated with a decreased BRS reactivity to postural stress (measured as an increased rBRSHF ratio). No significant association was found in the high AIRg group (Fig. 4b). The results in Fig. 4 were also corroborated by MLR analysis.
Discussion
While previous studies examined the associations between HRV and OSA severity,31,32,33 and between metabolic function and OSA severity,10,34 this study is the first to have measured all 3 factors—metabolic, autonomic and sleep—in the same subjects, using a variety of metrics to better characterize each factor. Moreover, this is the first study of its kind in a pediatric population, and follows an earlier report by our group that impaired glucose homeostasis in obese adolescents is correlated to sleep fragmentation and episodic hypoxemia.10 In this study, we employed a comprehensive battery of noninvasive measures of autonomic function, derived from HRV and BPV and their responses to orthostatic stress, as well as precise measures characterizing the dynamics of glucose–insulin regulation derived from the FSIVGTT in a relatively homogeneous group of otherwise healthy adolescent males with well characterized PSG parameters.
Our key findings may be summarized as follows:
-
1.
Reactivity of low-frequency HRV (rRRILF) to orthostatic stress was correlated with insulin sensitivity, independent of OSA severity.
-
2.
Baseline (supine) systolic BP (SBPsup) was higher in subjects with metabolic indices consistent with lower SI, greater IR (higher HOMAIR), increased fasting glucose, and reduced beta-cell function (lower DI), independent of OSA severity.
-
3.
Reactivity of baroreflex sensitivity to orthostatic stress was reduced with greater sleep fragmentation (as measured by arousal frequency), but only in those subjects with low SI and/or low first-phase insulin response to glucose (AIRg).
Figure 5 provides a schematic illustration of these key findings, along with those from our previous study,10 highlighting the interactions among the metabolic, autonomic, and sleep regulation systems.
Diagram illustrating the interactions found between autonomic function, OSA severity, and metabolic function. As mentioned in the text, the association between the reactivity index rRRILF and SI suggests sympathetic autonomic dysfunction to orthostatic stress predominantly for subjects with SI < 5 (mU/l)−1 min−1. Likewise, the association between increased sleep fragmentation and decreased vagal reactivity to postural stress (rBRSHF) was found to be significant only for those subjects with low insulin sensitivity (SI) and/or low acute first-phase insulin response to glucose (AIRg)
HRV and metabolic function
To better understand the meaning and implications of the above findings, it is important to review what is commonly accepted about the autonomic indices we extracted from noninvasive measurements of beat-to-beat RRI and BP. The spectral (frequency-based) content of HRV is commonly used to deduce useful information about the vagal and sympathetic contributions to HR modulation. RRIHF is generally ascribed to vagal modulation, whereas there is consensus that RRILF represents a combination of sympathetic and vagal modulation. The ratio of RRILF to RRIHF (=RRILHR) is generally referred to as the index representing “sympathovagal balance”. However, a high RRILHR could imply sympathetic-over-vagal dominance, or it could also reflect parasympathetic withdrawal with little contribution from the sympathetic branch. HRV indices measured during baseline conditions in the supine posture are expected to reflect parasympathetic dominance; during standing, the sympathetic activity increases while vagal or parasympathetic activity decreases. Our subjects indeed exhibited a significant vagal withdrawal during standing compared to supine, as indicated by a decrease in mRRI and RRIHF, and an increase in the ratio RRILHR, reflecting a shift to sympathetic dominance from supine to standing (Table 2). On the other hand, the change in RRILF from supine to standing was not significant. This may be explained by the fact that orthostatic stress leads to parasympathetic withdrawal (which decreases RRILF) and sympathetic excitation (which increases RRILF); thus, whether there is a net increase or decrease in RRILF, depends on which autonomic branch contributes more to the change in HRV following postural change.
None of the measures of HRV under baseline or standing conditions in our subjects were associated with any metabolic or OSA index. This could have been due to the high degree of inter-subject variability in these HRV indices, while none of the subjects had severe manifestations of metabolic dysfunction or OSA. As such, we turned to the “reactivity” indices instead, since these reflect changes in HRV in response to a strong autonomic stimulus (orthostatic stress) and therefore may have been more sensitive to metabolic or OSA effects. What stood out was the positive correlation between rRRILF (the reactivity index of RRILF to orthostatic stress) and SI (Fig. 1). Subjects with high SI tended to have rRRILF close to or greater than unity (and thus a net increase in RRILF from supine to standing), suggesting that these subjects had a more robust sympathetic contribution to the HR response to orthostatic stress. In contrast, in subjects with low SI, the increase in HR accompanying standing was likely dominated by vagal withdrawal—hence the net decrease in RRILF. A potential explanation is that, in subjects with low SI, the overall sympathetic contribution to HRV was already high—thus, orthostatic stress was less likely to elicit greater sympathetic modulation of HR.
BP and metabolic function
Although none of our subjects were diagnosed with Type 2 diabetes and all presented fasting glucose levels within the normal range (≤100 mg/dl), higher baseline SBPsup was correlated with higher fasting glucose, higher IR (as measured by the HOMAIR index), lower SI, and lower DI, all components of the metabolic syndrome.20 Given that there was no correlation between mRRI (or equivalently, mean HR) and any of the metabolic indices, we speculate that total peripheral resistance was the likely driver of the relationship between SBPsup and insulin resistance. This further suggests that subjects with low SI and higher fasting glucose probably had increased peripheral vascular sympathetic tone. Surprisingly, we found no independent correlation between BP and OSA severity, suggesting that the elevated peripheral vascular sympathetic activity in the subjects we studied was more likely the result of metabolic dysfunction than their OSA per se.
Baroreflex reactivity and metabolic-sleep interaction
While baseline (supine) systolic BP and reactivity of low-frequency HRV (rRRILF) correlated with the factors associated with the metabolic syndrome, but not to any measure of OSA severity, a third measure of autonomic function, the reactivity of BRS to orthostatic stress (rBRSHF), was correlated with sleep fragmentation (as measured by TAI). More importantly, our findings reveal that this rBRSHF–TAI correlation was only present in those subjects with low SI and/or low first-phase insulin response to glucose (AIRg), both manifestations of metabolic dysfunction. A number of previous studies have reported a blunted BRS in subjects with OSA,35,36,37 but these studies did not control for metabolic conditions. Since baroreflex sensitivity is believed to be a reliable measure of vagal tone,38 we interpret this result to indicate that sleep fragmentation leads to blunting of vagal reactivity to orthostatic stress in subjects with high IR and/or low AIRg. It has been well documented that arousals from sleep are generally accompanied by surges of sympathetic activity; thus, it is conceivable that blunting of vagal reactivity could result from cumulative exposure to these sympathetic bursts that occur with high-frequency night after night.
Inferences from the findings
As summarized in Fig. 5, the key findings of this study and our previous report10 on obese adolescents with OSA point to: (a) a strong association between measures of autonomic and metabolic function; (b) a robust association between measures of OSA severity and glucose metabolism; and (c) an association between autonomic function and OSA severity that is weak in subjects with metabolic health but enhanced in subjects with impaired glucose metabolism. This interaction between metabolic health and OSA severity has also been reported by Koren et al.39 In a study with both obese and nonobese children, the authors found that OSA severity, as measured by AHI, was associated with obesity, but only for those subjects who were more insulin resistant. However, this study did not measure autonomic function. The results from our study are also consistent with those of Cerutti et al.40 in which oral glucose tolerance tests were conducted and HRV was measured in three categories of subjects: obese girls with Type 2 diabetes, obese girls with no family history of diabetes, and lean age-matched controls.. However, Cerutti and colleagues did not include indices of sleep or OSA as a third factor. Peltier and colleagues41 found no differences in autonomic cardiovascular stress test results in adults with OSA versus subjects without OSA. However, when the same pool of subjects was divided into those who had impaired glucose regulation and those with normal glucose metabolism, the former group demonstrated impaired autonomic function. In overweight adult subjects with and without OSA, Wang et al.42 found that HRV and BRS determined from the polysomnogram across sleep stages correlated with the degree of glucose intolerance but not with the severity of OSA. These studies suggest that in overweight or obese subjects, metabolic dysfunction is the primary driver of autonomic imbalance, with OSA severity playing a secondary role. Insulin infusion has been shown to increase muscle sympathetic nerve activity (MSNA) in healthy and borderline hypertensive subjects,43,44 while treatment of hypertension does not reverse the IR.45 Thus, hyperinsulinemia, secondary to IR, appears to be the key driver of sympathetic overactivity.
The cross-sectional nature of our study does not permit a definitive conclusion on the causalities to explain the associations found among the variables investigated. Longitudinal studies with larger cohorts and carefully designed interventions are necessary to confirm our results. Nevertheless, taken together with other studies, our findings suggest that there is a need to control for metabolic conditions in investigations examining the contribution of OSA to autonomic dysfunction. Since most previous studies investigating cardiovascular autonomic function in OSA did not measure glucose or insulin, it is highly possible that the abnormalities in autonomic function reported in these studies were more directly related to metabolic derangements than to the repetitive arousals or intermittent hypoxia in OSA.
Conclusions
Our findings suggest that increased peripheral vascular sympathetic activity (indicated by increased baseline SBP) and reduced vagal autonomic reactivity to postural stress (as measured by rRRILF) are associated with manifestations of the metabolic syndrome, such as higher fasting glucose, higher insulin resistance, lower insulin sensitivity, and decreased compensatory β-cell function, but not with the severity of OSA. In other words, impairment of the sympathetic limb of autonomic function is more strongly affected by the level of metabolic dysfunction than by the severity of OSA in pediatric obesity. We speculate that metabolic dysfunction may be the initial entry point paving the way for other co-morbidities and thus provides an important early target for treatments in order to modify the progression of disease in obesity. Additionally, the ratio rBRSHF, related to vagal activity, was found to be negatively associated with sleep fragmentation present in OSA, but only for those subjects with low SI and low AIRg, both components of the metabolic syndrome. Thus, impairment in vagal reactivity to orthostatic stress in OSA is worsened when metabolic dysfunction is also present. The strong association with metabolic dysfunction suggests that the measurement of the autonomic reactivity to postural stress may be useful as a complementary noninvasive means for the early detection of the metabolic syndrome in young at-risk.
References
Martín-Timón, I., Sevillano-Collantes, C., Segura-Galindo, A. & Cañizo-Gómez, J. d. F. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J. Diabetes 5, 444–470 (2014).
Kohler, M. Risk factors and treatment for obstructive sleep apnea amongst obese children and adults. Curr. Opin. Allergy Clin. Immunol. 9, 4–9 (2009).
Gami, A. S. et al. Obstructive sleep apnea, obestiy, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 49, 565–571 (2007).
McNicholas, W. T. & Bonsignore, M. R. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanims and research priorities. Eur. Respir. J. 29, 156–178 (2007).
Ip, M. S. M. et al. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 165, 670–676 (2002).
Punjabi, N. M. & Polotsky, V. Y. Disorders of glucose metabolism in sleep apnea. J. Appl. Physiol. 99, 1998–2007 (2005).
Chasens, E. R., Weaver, T. E. & Umlauf, M. G. Insulin resistance and obstructive sleep apnea: is increased sympathetic stimulation the link?. Biol. Res. Nurs. 5, 87–96 (2003).
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Executive Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol Education Program (Adult Treatment Panel III). JAMA, 285, 2486–2497 (2001).
American Diabetes Association. Type 2 diabetes in children and adolescents. Pediatrics 105, 671–680 (2000).
Lesser, D. J. et al. Sleep fragmentation and intermittent hypoxemia are associated with decreased insulin sensitivity in obese adolescent Latino males. Pediatr. Res. 72, 293–298 (2012).
Shaibi, G. Q. & Goran, M. I. Examining metabolic syndrome definitions in overweight Hispanic youth: a focus on insulin resistance. J. Pediatr. 152, 171–176 (2008).
Goran, M. I., Bergman, R. N., Cruz, M. L. & Watanabe, R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care 25, 2184–2190 (2002).
Cruz, M. L. et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J. Clin. Endocrinol. Metab. 89, 108–113 (2004).
Berry, R. B. et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for scoring of sleep and associated events. J. Clin. Sleep. Med. 8, 597–619 (2012).
Witmans, M. B., Keens, T. G., Davidson Ward, S. L. & Marcus, C. L. Obstructive hypopneas in children and adolescents. Am. J. Respir. Crit. Care Med. 168, 1540 (2003).
Kang, K.-T. et al. Impacts of disease severity on postoperative complications in children with sleep-disordered breathing. Laryngoscope 127, 2646–2652 (2017).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Galvin, P. et al. A simple method for quantitation of insulin sensitivity and insulin release from an intravenous glucose tolerance test. Diabet. Med. 9, 921–928 (1992).
Punjabi, N. M. & Beamer, B. A. Alterations in glucose disposal in sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 179, 235–240 (2009).
Khoo, M. C. K., Oliveira, F. M. G. S. & Cheng, L. Understanding the metabolic syndrome: a modeling perspective. IEEE Rev. Biomed. Eng. 6, 143–155 (2013).
Bergman, R. N., Ider, Y. Z., Bowden, C. R. & Cobelli, C. Quantitative estimation of insulin sensitivity. Am. J. Physiol. 236, E667–E677 (1979).
Carnethon, M. R. et al. Does the cardiac autonomic response to postural change predict incident coronayr heart disease and mortality? The atherosclerosis risk in communities study. Am. J. Epidemiol. 155, 48–56 (2002).
Swenne, C. A. Baroreflex sensitivity: mechanisms and measurement. Neth. Heart J. 21, 58–60 (2013).
Pinna, G. D., Maestri, R., Raczak, G. & La Rovere, M. T. Measuring baroreflex sensitivity from the gain function between arterial pressure and heart period. Clin. Sci. 103, 81–88 (2002).
Goran, M. I., Shaibi, G. Q., Weigensberg, M. J., Davis, J. N. & Cruz, M. L. Deterioration of insulin sensitivity and beta-cell function in overweight Hispanic children during pubertal transition: a longitudinal assessment. Int J. Pediatr. Obes. 1, 139–145 (2006).
Hassard, T. H. Understanding Biostatistics (Mosby-Year Book, St. Louis, MO, 1991).
Chatelain, J.-B. & Ralf, K. Spurious regressions and near-multicollinearity, with an application to aid, policies and growth. J. Macroecon. 39, 85–96 (2014).
Stewart, J. M. Mechanisms of sympathetic regulation in orthostatic intolerance. J. Appl. Physiol. 113, 1659–1668 (2012).
Pagani, M. et al. Assessment of the neural control of the circulation during psychological stress. J. Auton. Nerv. Syst. 35, 33–42 (1991).
Malliani, A. et al. Spectral analysis to assess increased sympathetic tone in arterial hypertension. Hypertension 4(suppl III), 36–42 (1991).
Enright, P. L., Goodwin, J. L., Sherrill, D. L., Quan, J. R. & Quan, S. F. Blood pressure elevation associated with sleep-related breathing disorder in a community sample of white and hispanic children: the Tucson Children’s Assessment of Sleep Apnea Study. Arch. Pediatr. Adolesc. Med. 157, 901–904 (2003).
Nisbet, L. C., Yiallourou, S. R., Walter, L. M. & Horne, R. S. C. Blood pressure regulation, autonomic control and sleep disordered breathing in children. Sleep. Med. Rev. 18, 179–189 (2014).
Eyck, A. V. et al. Sleep disordered breating and autonomic function in overweight and obese children and adolescents. ERS Open Res. 2. https://doi.org/10.1183/23120541.00038-2016 (2016).
Bhushan, B. et al. Metabolic alterations in children with obstructive sleep apnea. Int J. Pediatr. Otorhinolaryngol. 78, 854–859 (2014).
Carlson, J. T., Hedner, J. A., Sellgren, J., Elam, M. & Wallin, G. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am. J. Resp. Crit. Care Med. 154, 1490–1496 (1996).
Narkiewicz, K. et al. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension 32, 1039–1043 (1998).
Jo, J. A. et al. Determinants of heart rate variability in obstructive sleep apnea syndrome during wakefulness and sleep. Am. J. Physiol. Heart Circ. Physiol. 288, H1103–H1112 (2005).
Casadei, B., Meyer, T. E., Coats, A. J. S., Conway, J. & Sleight, P. Baroreflex control of stroke volume in man: an effect mediated by the vagus. Am. J. Physiol. Cell Physiol. 448, 539–550 (1992).
Koren, D., Gozal, D., Philby, M. F., Bhattacharjee, R. & Kheirandish-Gozal, L. Impact of obstructive sleep apnoea on insulin resistance in nonobese and obese children. ERJ Express 52. https://doi.org/10.1183/13993003.01430-2015, 2015.
Cerutti, F. et al. Impairment in cardiovascular autonomic pattern in obese adolescents with Type 2 diabetes mellitus. J. Endocrinol. Invest. 33, 539–543 (2010).
Peltier, A. C. et al. Autonomic dysfunction in obstructive sleep apnea is associated with impaired glucose regulation. Sleep. Med. 8, 149–155 (2007).
Wang, W., Redline, S. & Khoo, M. C. K. Autonomic markers of impaired glucose metabolism: effects of sleep disordered breathing. J. Diabetes Sci. Technol. 6, 1–13 (2012).
Berne, C., Fagius, J., Pollare, T. & Hjemdahl, P. The sympathetic response to euglycaemic hyperinsulinaemia: the evidence from microelectrode nerve recordings in healthy subjects. Diabetologia 35, 873–879 (1992).
Anderson, E. A., Balon, T. W., Hoffman, R. P., Sinkey, C. A. & Mark, A. L. Insulin increases sympathetic activity but not blood pressure in borderline hypertensive humans. Hypertension 19, 621–627 (1992).
Reaven, G. M. Relationship between insulin resistance and hypertension. Diabetes Care 14, 33–38 (1991).
Funding
This work was supported in part by NIH Grants HL105210, HL090451, EB001978, RR00047, and the USC Center for Transdisciplinary Research on Energetics and Cancer (TREC U54 CA 116848). F.M.S. Oliveira also thanks the Fulbright/CAPES program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Oliveira, F.M.S., Tran, W.H., Lesser, D.J. et al. Abnormalities in autonomic function in obese boys at-risk for insulin resistance and obstructive sleep apnea. Pediatr Res 85, 790–798 (2019). https://doi.org/10.1038/s41390-018-0226-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0226-2
This article is cited by
-
Poverty and chronic illness: why safety net programs matter
Pediatric Research (2019)