Abstract
Background and Objectives
Sepsis leads to systemic inflammatory response with cerebral blood flow (CBF) alteration and blood–brain barrier disruption that contribute to sepsis-associated encephalopathy (SAE). We aimed to evaluate cord blood neuron-specific enolase (cNSE) and CBF in early-onset neonatal sepsis (EONS) as predictors of SAE and to define short-term neurodevelopmental outcomes among survivors.
Methods
cNSE was measured in 200 neonates with antenatal risk factors for EONS, stratified into two groups: sepsis (n = 96) and no-sepsis (n = 104). Trans-cranial Doppler of peak systolic velocities (PSV), end diastolic velocities (EDV) and resistive indices (RI) of anterior (ACA) and middle (MCA) cerebral arteries recorded on day 1 postnatal. Griffiths mental developmental scale (GMDS) was assessed at 6 months.
Results
Increased cNSE, PSV, EDV, and decreased RI of both ACA and MCA were found in sepsis group compared to no-sepsis group (p < 0.001 for all). Patients with SAE (n = 34) had higher NSE, PSV, and EDV as well as lower RI of ACA and MCA compared to those without (p < 0.01 for all). SAE neonates had lower GMDS than those without. ACA RI of ≤0.61 was the best predictor of SAE.
Conclusion
High CBF and cNSE could be useful markers for prediction of SAE. SAE impairs neurodevelopmental scales at 6 months.
Similar content being viewed by others
Introduction
Neonatal sepsis is one of the leading causes of morbidity and mortality among term and preterm infants worldwide.1 Although advances in neonatal care have improved survival and reduced complications, survivors of neonatal sepsis are still vulnerable to short- and long-term neurodevelopmental morbidity.2
Early-onset neonatal sepsis (EONS) results in an extremely complex chain of events involving pro-inflammatory and anti-inflammatory processes, humoral and cellular reactions, and circulatory abnormalities.3
Sepsis-associated encephalopathy (SAE) is defined as diffuse cerebral dysfunction induced by the systemic inflammatory response without overt central nervous system infection.4, 5 Clinical presentation of SAE may range from a transient, reversible encephalopathy to irreversible brain damage.6 SAE is associated with a poor outcome.7
The pathophysiology of SAE is complex and multifactorial including a number of mechanisms such as the local generation of pro-inflammatory cytokines, alterations in cerebral blood flow velocities (CBFV),8 an imbalance of neurotransmitters, peripheral organ failure, 9 and damage of the blood–brain barrier (BBB)4 that lead to the release of neuro-biochemical protein markers, such as neuron-specific enolase (NSE) into the circulation.10
There is paucity of literature investigating the CBF alterations and the release of neuroinflammatory biomarkers in neonatal sepsis and sepsis-associated encephalopathy. The present study aimed to evaluate cord blood neuron-specific enolase (cNSE) levels and CBF measurements in early-onset neonatal sepsis (EONS) as predictors of SAE. Secondary aim was to examine short-term neurodevelopmental outcomes among SAE neonates.
Patients and methods
Study population
This prospective observational cohort study was carried out at two neonatal intensive care units (NICUs), Ain Shams University Hospitals from December 2014 till December 2016. The mothers of the newborns provided written consent for the protocol which was approved by the Research Ethical Committee of Ain Shams University hospitals; ID: FMASU 1601/2013 and are in accordance with the Helsinki Declaration of 1975.
This study included any neonate having one or more maternal risk factors for EONS; evidence of clinical chorioamnionitis, foul smelling liquor, antepartum/intrapartum maternal fever,11 prolonged and premature rupture of membranes ≥18 h, offensive vaginal discharge and/or maternal blood leucocytosis (>15,000 leucocytes/mL).12 Typically clinical chorioamnionitis is diagnosed by the presence of fever >37.8 °C in addition to two other signs (uterine tenderness, maternal or fetal tachycardia, and foul/purulent amniotic fluid).13
Neonates with major congenital birth defects or chromosomal anomalies, perinatal asphyxia, hemodynamically significant cardiac abnormalities, requiring mechanical ventilation, or receiving drugs like vasopressors, caffeine, theophylline, or anticonvulsants at the time of CBF velocity estimation were excluded from the study.
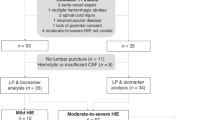
Out of 244 neonates assessed for eligibility, 44 were excluded: 36 did not meet inclusion criteria and 8 guardians declined to participate. Thus, 200 consecutively inborn neonates were enrolled. The included neonates were divided into 2 distinct groups; 96 neonates with established diagnosis of EONS based on the development of clinical evidence suggestive of sepsis within 72 h of birth,14 quantitative C-reactive protein (CRP) levels ≥6 mg/L, Rodwell’s hematological scores ≥315 and/or Tollner’s scores ≥10,16 with or without positive blood culture results, and 104 non-sepsis neonates in whom EONS was ruled-out on the basis of absence of any clinical or laboratory evidence suggestive of infection.
Clinical examination and routine neonatal care
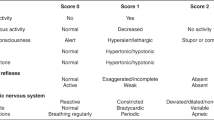
Detailed perinatal history was obtained for all neonates, followed by a thorough clinical examination. The gestational age was determined by maternal last menstrual period and further confirmed by using the Ballard score.17 Birth anthropometric parameters including birth weight, birth length, and occipito-frontal circumference were measured. All neonates received routine neonatal care according to our NICU protocol. SAE was defined by difficulty with initiating and maintaining respiration, depression of tone and reflexes, subnormal level of consciousness, and/or seizures in absence of central nervous system infection.18
Neurodevelopmental assessment
Routine musculoskeletal examination was performed at 6 months of age as well as neurodevelopmental assessment using the Griffiths mental developmental scales (GMDS) for sepsis survivors (n = 66) at 6 months postnatal age, done by the same observer who was blinded to patient group, assessing 5 areas of development: locomotor (subscale A), personal-social (subscale B), hearing and language (subscale C), eye and hand co-ordination (subscale D), and performance (subscale E). The number of items passed in each subscale is counted up and the totals are entered in a printed form to get a final score: General Intelligence Quotient (GQ), Intelligence quotients of A subscale (AQ), Intelligence Quotients of B subscale (BQ), Intelligence Quotients of C subscale (CQ), Intelligence Quotients of D subscale (DQ), and Intelligence Quotients of E subscale (EQ).19
Laboratory analysis
For complete blood count (CBC) analysis, cord blood samples were obtained on potassium-ethylene diamine tetra acetic acid (K2-EDTA) in sterile vacutainers, and right after collection, the tube was gently inverted several times and placed into a cold box at 4–8 °C. The sample was transported and analysed on the same day. CBC was performed using the automated haematology analyser; Sysmex XT-1800i (Sysmex, Kobe, Japan). The XT-1800i performs analysis of WBCs with an optical detector based on the flowcytometry method. RBCs and platelet count analysis is done by the RBC detector using the Hydro Dynamic Focusing method.
For analysis of C-reactive protein (CRP) and NSE, cord blood samples were obtained on gel tubes without addition of anticoagulant and centrifuged for 15 min for separation of the serum. They were stored at −70 °C till assay. Repeated freeze-thaw cycles were avoided. Serum NSE assay was repeated on day 5 of life.
CRP levels were measured using AVITEX CRP; a rapid latex agglutination test kit (CRP Omega Diagnostics Ltd. Omega House, Scotland, United Kingdom). The AVITEX CRP latex particles are coated with antibodies to human CRP. When the latex suspension is mixed with serum containing elevated CRP levels on a slide, clear agglutination is seen within 2 min. Serial dilution was performed according to manufacturer instructions. The detection limit for CRP is 6 mg/L.
Blood cultures were done soon after birth using BD BACTEC PEDS PLUS/F culture vials (BENEX Limited, Shannon, County Clare, Ireland Ireland). Blood culture samples were collected into the culture bottles, transported immediately to the laboratory, and processed using the BD BACTEC FX40 systems (Becton–Dickinson Microbiology Systems, Sparks, MD). A single blood culture bottle was used per patient. The minimal volume inoculated was 2 mL (3 mL was an ideal volume) and blood culture bottles were incubated for a maximum of 5 days (unless they flag positive). The BD BACTEC FX40 system detects positive cultures based on CO2 production. Blood culture bottles which flagged positive were cultured on standard media with the use of routine microbiological techniques. Analytical Profile Index (API) biochemical test kit (BioMerieux, France) were used to confirm suspected pathogens.
The level of NSE in the samples was determined using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) kit supplied by DiaMetra, Paciana, Italy (REF DKO073). In this technique, samples or standards containing NSE were captured between two antibodies: the first was fixed to the inner wall of ELISA wells plate and the second was labeled with biotin to which horse radish peroxidase was combined forming immune complex. Addition of the TMB Substrate solution initiates a kinetic reaction which leaded to color development. Color development was terminated by the addition of the Stop Solution after a certain time as mentioned by the manufacturer. The color developed was measured spectrophotometrically at a wavelength of 450 nm. The concentration of NSE was proportional to the color intensity of the test sample. A standard curve was constructed from which the concentrations of NSE in the samples were determined. Cerebrospinal fluid (CSF) analysis including culture was done in all septic neonates.
Trans-cranial ultrasound assessment
Trans-cranial ultrasound (TCUS) was carried out by the same ultrasonologist, who was blinded to the patient group, for assessment of blood flow velocity in the anterior (ACA) and middle cerebral arteries (MCA) using duplex pulsed Doppler ultrasound within the first 24 h postnatal. Peak systolic velocity (PSV), end diastolic velocity (EDV), and resistive index (RI) were measured. All the TCUS studies were done in thermo-neutral environment in quiet neonates using the two dimensional/pulsed and color Doppler Ultrasound (LOGIQ 400 proseries General electric, Ultramark 9 Color Doppler System, Advanced Technology Laboratories, Bothell, WA) with a 5 MHz transducer. Using the anterior fontanel as an acoustic window, CBF velocity was measured in the ACA with the angle of insonation less than 10°. MCA was accessed through transtemporal windows.20 PSV and EDV were calculated from at least three consecutive cardiac cycles of optimal quality using the built-in calculation program. RI of ACA and MCA were automatically calculated after recording both PSV and EDV according to the Pourcelot index of resistance.21 TCUS was repeated during the hospital stay for intracranial hemorrhage (ICH) screening.
Primary and secondary outcomes
Primary outcome includes assessment of the changes in CBF velocity and serum NSE. Secondary outcomes include length of hospital stay, use and duration of drug intake as antibiotics and inotropes, use and duration of mechanical ventilation, evidence of neonatal encephalopathy, evidence of multisystem organ dysfunction (MSOD) characterized by the development of progressive and potentially reversible physiologic dysfunction in 2 or more organs or organ systems that is induced by a variety of acute insults including sepsis,22 neurodevelopmental assessment using GMDS, and mortality.
Sample size calculation
Sample size was calculated using MedCalc© version 14 (MedCalc© Software bvba, Ostend, Belgium), setting the type-1 error (α) at 0.05 and the power (1 − β) at 0.8. Basu et al.23 study showed that the area under the curve of different cerebral blood flow velocity parameters in EONS for prediction of SAE ranged between 0.846 and 1, with 25% incidence of encephalopathy among sepsis cases. Accordingly, a sample of at least 94 neonates per group (total 188) was calculated.
Statistical analysis
Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version 22 (SPSS Inc., Armonk, NY).
Normality of numerical data distribution was examined using the Shapiro–Wilk test. Normally distributed numerical variables were presented as mean (SD) and inter-group differences were compared using the unpaired t-test. Skewed numerical variables were presented as median (interquartile range), and between-group differences were compared using the Mann–Whitney test.
Categorical variables were presented as number (%), and differences between groups were compared using Fisher’s exact test. Ordinal data were compared using the χ2 test for trend. The comparison between two paired parameters with non-parametric data were done by using Wilcoxon rank test. Correlations among numerical variables were examined parametrically using the Pearson product–moment correlation, or non-parametrically using the Spearman rank correlation, as appropriate.
Logistic regression analysis was used to assess the predictors of SAE from the cerebral Doppler indices and cNSE. Receiver-operating characteristic (ROC) curve analysis was used to examine the value of continuous variables for prediction of SAE. To assess the overall accuracy of the ROC analyses, the area under the curves (AUCs) was measured. As the AUC increases towards 1.00, its usefulness as a diagnostic tool is seen to increase. The DeLong method was used to compare the areas under individual ROC curves. A p-value < 0.05 was considered statistically significant.
Results
As shown in Table 1, there was no significant difference between sepsis and non-sepsis group as regards maternal characteristics or neonatal demographic data. Neonatal anthropometric measurements showed that the sepsis group had lower birth weight than the non-sepsis group (2356 ± 760 vs. 2653 ± 723, p = 0.048). In the view of cerebral Doppler indices, sepsis group had statistically significant lower resistive indices and higher PSV and EDV of ACA and MCA than the non-sepsis group (p < 0.001). Also, the sepsis group had a statistically significant higher cNSE than the non-sepsis group (p < 0.001).
The blood culture was positive among 44 septic neonates (45.8%) with Staphylococcus aureus documented in 10 neonates (10.4%), Coagulase negative staphylococci in 8 neonates (8.3%), Citrobacter in 8 neonates (8.3%), Acinetobacter in 6 neonates (6.3%), gram positive streptococci in 4 neonates (4.2%), Klebsiella in 4 neonates (4.2%), Neisseria species in 2 neonates (2.1%), and Streptococcus pneumonia in 2 neonates (2.1%). CRP median (IQR) was 6(0–12) mg/L, Tollner’s score median (IQR) was 11(10-12) and Rodwell’s score median (IQR) was 4(3-5) None of our patient developed meningitis and all of them had sterile CSF culture.
Table 2 showed that SAE neonates (n = 34) had statistically significant lower resistive indices and higher PSV and EDV of ACA and MCA than sepsis without encephalopathy (n = 62) (p < 0.001 for all). Also, SAE neonates had statistically significant higher cNSE and follow-up NSE than those without encephalopathy (p = 0.039, p = 0.001; respectively). Moreover, in neonates with SAE, cord blood NSE levels were significantly higher than the follow-up levels [150 (92.38–190.5) vs. 93.15 (65.65–102.95); p = 0.001]. Furthermore, GMDS done for SAE survivors at 6 months postnatal age (n = 23) neonates had significantly lower GMDS than those did not had encephalopathy (n = 43); (p < 0.01 for all; Fig. 1).
Griffiths mental developmental scales indices at 6 months of age in septic neonates with and without encephalopathy. AQ: Intelligence quotients of A subscale, BQ: Intelligence quotients of B subscale, CQ: Intelligence quotients of C subscale, DQ: Intelligence quotients of D subscale, EQ: Intelligence quotients of E subscale, GQ: General Intelligence quotient. Data were expressed as mean ± SD. Independent t-test showed statistically significant difference among all groups
Logistic regression analysis showed that cNSE and ACA RI were the significant independent variables that could predict SAE. ROC curve analysis revealed that ACA RI had the best AUC; 0.928 with a cutoff point ≤0.61 that has 85.7% sensitivity and 84.6% specificity to predict SAE.
As regards secondary outcome measures, Table 3 showed that the sepsis group had statistically significant higher length of hospital stay (p < 0.001), higher use and duration of respiratory support (p < 0.001), use and duration of inotropes (p < 0.0001), longer duration of antibiotics (p < 0.001), higher incidence of encephalopathy (p < 0.001), higher incidence of MSOD (p < 0.001), higher percentage of abnormal TCUS findings (p = 0.003), and higher mortality (p < 0.001).
Correlation studies showed significant positive relations between cNSE and ACA PSV, ACA EDV, MCA PSV, and MCA EDV in sepsis group (p-value < 0.05 for all). However, no significant correlations were found between ACA and MCA Doppler indices with GMDS indices at 6 months among the sepsis group (p-value > 0.05 for all; Supplementary table S1). Also, no significant correlations were found between cNSE and GMDS indices at 6 months among the sepsis group (p-value > 0.05 for all; Supplementary table S2).
Discussion
Systemic infection remains a major cause of morbidity and mortality in the neonatal period. Clinical and experimental data suggest that changes in cerebral blood flow, release of neuroinflammatory proteins and metabolic alterations contribute to sepsis-associated neuronal dysfunction.6, 9 There are no precise, well-established clinical, or biological markers of sepsis-induced brain injury.7
In the current study, we found that septic neonates had lower birth weight than the no-sepsis group. Similarly, Krajčinović et al.24 observed that neonatal sepsis occurs most frequently in newborns with lower birth weight, also Wynn and Levy25 reported that infant’s birth weight is inversely related to risk of EONS.
Detecting organisms in blood cultures is the gold standard for diagnosis of septicemia,26 though not all cultures taken from septic neonates show positive results.27 In the present analysis, 44 out of 96 septic neonates (45.8%) had positive blood culture. Another study from Egypt documented culture proven sepsis in 40.7% of the examined neonates.28 This is comparable to rates reported in other developing African and Asian countries as Nigeria (45.9%).29
Upon studying the effect of sepsis on CBFV, sepsis group showed a significant increase in CBFV with lower resistive indices and higher PSV and EDV of both ACA and MCA than the no-sepsis group. Moreover, within the sepsis group, we observed that CBFV was significantly increased in those who had SAE compared to those who did not and that the ACA RI ≤ 0.61 could predict SAE with 85.7% sensitivity, 84.6% specificity and AUC was 0.928. In agreement with our findings, Basu et al.23 reported significantly lower RI and pulsatility index, vasodilatation, and higher PSV in MCA in neonates with EONS within 24 h of birth.
Abnormal cerebral hemodynamics in sepsis has been explained by many theories; injured BBB allows high levels of endogenous catecholamines to directly affect cerebral vascular resistance,30 impaired endothelial nitric oxide synthase resulting in altered cerebrovascular hemodynamics,6 also changes in the coagulation system resulting in microthromboses and microinfarcts contribute to the microcirculatory dysfunction.31
Examining NSE as a biomarker of sepsis and sepsis-induced cerebral injury revealed that the cNSE was higher in sepsis group compared to the no-septic group. A similar trend was observed by Hsu et al.10 who reported elevated serum NSE in patients with septic shock compared to the control group.
In the current analysis, we found that cNSE in sepsis neonates who developed encephalopathy was higher than those who did not develop SAE and that cNSE was one of the significant independent variables that could predict SAE. Moreover, follow-up NSE withdrawn from neonates with SAE was higher than those of sepsis neonates without encephalopathy. Furthermore, there was a significant association between cNSE and the systolic and diastolic CBFV in the ACA and MCA in sepsis group.
Our findings support those of Yao et al.32 who found that serum NSE in SAE patients was significantly higher than in non-SAE patients. Additionally, a systematic review was done to define the role of NSE for diagnosing and monitoring SAE in which seven studies identified a positive association between elevated levels of NSE and the development of SAE.33
More analysis of the short-term effect of EONS on the neonatal neurodevelopment and cognitive functions, the current study showed that SAE survivors had significantly lower GMDS at 6 months than septic neonates without encephalopathy. Similarly, Dammann et al.34 documented that there was a relationship between antenatal infection and cognitive limitations. Kohlendorfer et al.35 also found that children who had neonatal sepsis were three times more likely to have neuromotor and cognitive development impairment at 12 months of age.
In the view of the effect of sepsis on different organs or organ systems, we observed a significantly higher need for respiratory support, use of inotropes, higher incidence of encephalopathy and MSOD in sepsis group. Progressive MSOD is the most common cause of death in sepsis, with the lung usually representing the first organ to fail.36 This can be the result of lung vascular endothelial injury with increased permeability, leading to interstitial and alveolar edema, neutrophil entrapment, and the injury to alveolar capillary membranes leading to acute lung injury and acute respiratory distress syndrome.37 Moreover, many pro-inflammatory cytokines are produced and released in bacteremia leading to a cascade of inflammatory reaction with production of nitrogen and oxygen reactive species that cause additional endothelial damage, injury in the intercellular junctions causing edema, vasodilation, and lost vascular tone.38
On an additional attempt to evaluate the secondary outcomes of EONS, we found that sepsis group had longer length of hospital stay, duration of antibiotics, more ICH, and higher mortality than no-sepsis group. Similarly, Basu et al.23 reported that 14.5% of the sepsis group showed signs of ICH. Moreover, Rocha et al.39 stated that periventricular–intraventricular hemorrhage is a well-known complication of chorioamnionitis. Hornik et al.40 reported that EONS was associated with an increased risk of death on multivariable analysis (OR = 1.45 [95% CI 1.21, 1.73]). Furthermore, Shehab El-Din et al.28 documented 51% mortality among septic neonates in 3 NICUs in Egypt.
Limitation of this study is that we followed our patients only for 6 months, we might have needed a longer duration of neurodevelopmental follow-up to detect affection in school achievements or executive and cognitive functions. Therefore, the study in its current form is not powered to use cNSE or doppler indices to predict risk for poor long-term neurodevelopmental outcomes.
In conclusion, the present study speculates that increased cerebral blood flow parameters in neonates with EOS within the earliest hours from birth could be used with a high predictive accuracy for detection of SAE. Cord blood NSE could also be a useful biomarker for prediction of sepsis-induced cerebral injury. SAE was found to significantly impair neurodevelopmental scales at 6 months of age. Future longitudinal studies are required to determine the impact of SAE on the infant’s long-term neurodevelopmental outcome.
Study Highlights
What is known
-
Neonatal sepsis is a leading cause of morbidity and mortality among term and preterm infants. Although advances in neonatal care have improved survival and reduced complications, the survivors of neonatal sepsis are still vulnerable to short- and long-term neurodevelopmental morbidity.
What this study adds
-
Increased cord blood NSE and cerebral blood flow in early hours of birth could be used in neonates with early-onset sepsis with a high predictive accuracy of sepsis-associated encephalopathy.
-
Sepsis-associated encephalopathy significantly impairs neurodevelopmental scales at 6 months of age.
References
Camacho-Gonzalez, A., Spearman, P. W. & Stoll, B. J. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr. Clin. North Am. 60, 367–389 (2013).
Ferreira, R. C., Mello, R. R. & Silva, K. S. Neonatal sepsis as a risk factor for neurodevelopmental changes in preterm infants with very low birth weight. J. Pediatr. 90, 293–299 (2014).
Gullo, A., Bianco, N. & Berlot, G. Management of severe sepsis and septic shock: challenges and recommendations. Crit. Care Clin. 22, 489–501 (2006).
Chaudhry, N. & Duggal, A. K. Sepsis associated encephalopathy. Adv. Med. 2014, 762320 (2014).
Cretin, B., Collongues, N., Philippi, N. & Blanc, F. Sepsis associated encephalopathy. Rev. Neurol. 167, 195–204 (2011).
Wilson, J. X. & Young, G. B. Progress in clinical neurosciences: sepsis-associated encephalopathy: evolving concepts. Can. J. Neurol. Sci. 30, 98–105 (2003).
Zampieri, F. G., Park, M., Machado, F. S. & Azevedo, L. C. Sepsis-associated encephalopathy: not just delirium. Clinics 66, 1825–1831 (2011).
Pytel, P. & Alexander, J. J. Pathogenesis of septic encephalopathy. Curr. Opin. Neurol. 22, 283–287 (2009).
Semmler, A. et al. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J. Neuroinflamm. 5, 38 (2008).
Hsu, A. A. et al. Neurological injury markers in children with septic shock. Pediatr. Crit. Care. Med. 9, 245–251 (2008).
Snyder, M., Crawford, P., Jamieson, B. & Neher, J. O. What treatment approach to intrapartum maternal fever has the best fetal outcomes? J. Fam. Pract. 56, 401–402 (2007).
Tita, A. T. N. & Andrews, W. W. Diagnosis and management of clinical chorioamnionitis. Clin. Perinatol. 37, 339 (2010).
Newton, E. R. Chorioamnionitis and intraamniotic infection. Clin. Obstet. Gynecol. 36, 795–808 (1993).
Chiesa, C., Panero, A., Osborn, J. F., Simonetti, A. F. & Pacifico, L. Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin. Chem. 50, 279–287 (2004).
Rodwell, R. L., Leslie, A. L. & Tudehope, D. I. Early diagnosis of neonatal sepsis using a hematologic scoring system. J. Pediatr. 112, 761–767 (1988).
Tollner, U. Early diagnosis of septicemia in the newborn—clinical-studies and sepsis score. Eur. J. Pediatr. 138, 331–337 (1982).
Ballard, J. L. et al. New ballard score, expanded to include extremely premature-infants. J. Pediatr. 119, 417–423 (1991).
Goldsmith, J. P. & Papile, L.-A. Updated monograph outlines advances in diagnosis, pathophysiology, treatment of neonatal encephalopathy. AAP News 35, 18 (2015).
Griffiths, R. Griffiths Mental Development Scales—Revised: Birth to 2 years (GMDS 0-2) (University of London Press, London, 1954).
Couture, A., Veyrac, C., Baud, C., Saguintaah, M. & Ferran, J. L. Advanced cranial ultrasound: transfontanellar Doppler imaging in neonates. Eur. Radiol. 11, 2399–2410 (2001).
Allison, J. W. et al. Intracranial resistive index (RI) values in normal term infants during the first day of life. Pediatr. Radiol. 30, 618–620 (2000).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315, 801–810 (2016).
Basu, S., Dewangan, S., Shukla, R. C., Anupurva, S. & Kumar, A. Cerebral blood flow velocity in early-onset neonatal sepsis and its clinical significance. Eur. J. Pediatr. 171, 901–909 (2012).
Krajcinovic, S. S., Doronjski, A., Barisic, N. & Stojanovic, V. Risk factors for neonatal sepsis and method for reduction of blood culture contamination. Malawi Med. J. 27, 20–24 (2015).
Wynn, J. L. & Levy, O. Role of innate host defenses in susceptibility to early-onset neonatal sepsis. Clin. Perinatol. 37, 307–337 (2010).
Bizzarro, M. J., Raskind, C., Baltimore, R. S. & Gallagher, P. G. Seventy-five years of neonatal sepsis at Yale: 1928-2003. Pediatrics 116, 595–602 (2005).
Bourchier, D. & Weston, P. J. Randomised trial of dopamine compared with hydrocortisone for the treatment of hypotensive very low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 76, F174–F178 (1997).
Shehab El-Din, E. M., El-Sokkary, M. M., Bassiouny, M. R. & Hassan, R. Epidemiology of neonatal sepsis and implicated pathogens: a study from Egypt. Biomed. Res. Int. 2015, 509484 (2015).
Meremikwu, M. M., Nwachukwu, C. E., Asuquo, A. E., Okebe, J. U. & Utsalo, S. J. Bacterial isolates from blood cultures of children with suspected septicaemia in Calabar, Nigeria. BMC Infect. Dis. 5, 110 (2005).
MacKenzie, E. T., McCulloch, J., O’Kean, M., Pickard, J. D. & Harper, A. M. Cerebral circulation and norepinephrine: relevance of the blood–brain barrier. Am. J. Physiol. 231, 483–488 (1976).
Vincent, J. L. Microvascular endothelial dysfunction: a renewed appreciation of sepsis pathophysiology. Crit. Care 5, S1–S5 (2001).
Yao, B., Zhang, L. N., Ai, Y. H., Liu, Z. Y. & Huang, L. Serum S100beta is a better biomarker than neuron-specific enolase for sepsis-associated encephalopathy and determining its prognosis: a prospective and observational study. Neurochem. Res. 39, 1263–1269 (2014).
Zenaide, P. V. & Gusmao-Flores, D. Biomarkers in septic encephalopathy: a systematic review of clinical studies. Rev. Bras. Ter. Intensiv. 25, 56–62 (2013).
Dammann, O., Kuban, K. C. & Leviton, A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment. Retard. Dev. Disabil. Res. Rev. 8, 46–50 (2002).
Kiechl-Kohlendorfer, U., Ralser, E., Pupp Peglow, U., Reiter, G. & Trawoger, R. Adverse neurodevelopmental outcome in preterm infants: risk factor profiles for different gestational ages. Acta Paediatr. 98, 792–796 (2009).
Ramirez, M. Multiple organ dysfunction syndrome. Curr. Probl. Pediatr. Adolesc. Health Care 43, 273–277 (2013).
Harrois, A., Huet, O. & Duranteau, J. Alterations of mitochondrial function in sepsis and critical illness. Curr. Opin. Anaesthesiol. 22, 143–149 (2009).
Short, M. A. Linking the sepsis triad of inflammation, coagulation, and suppressed fibrinolysis to infants. Adv. Neonatal Care. 4, 258–273 (2004).
Rocha, G., Proenca, E., Quintas, C., Rodrigues, T. & Guimaraes, H. Chorioamnionitis and brain damage in the preterm newborn. J. Matern. Fetal Neonatal Med. 20, 745–749 (2007).
Hornik, C. P. et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum. Dev. 88(Suppl 2), S69–S74 (2012).
Author information
Authors and Affiliations
Contributions
M.S.E.S. and R.A.E.F. conceptualized and designed the study. N.M.E.R. and H.A.S. contributed to the conceptualization and drafted the initial manuscript. H.E.M. and D.H.S. contributed to the study design and performed Doppler ultrasonography. M.A.A.S. and N.M.B. supervised data collection, laboratory investigations, and analyzed and interpreted the data. A.S.F. contributed to the study design, selection of obstetric patients and diagnosis of maternal chorioamnionitis, and analyzed and interpreted the data. A.K.E.Z. contributed to the conceptualization, performed Griffiths mental developmental scale, and neurological examination at 6 months of age. All authors contributed to data interpretation and manuscript writing and have read and approved the final submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
El Shimy, M.S., El-Raggal, N.M., El-Farrash, R.A. et al. Cerebral blood flow and serum neuron-specific enolase in early-onset neonatal sepsis. Pediatr Res 84, 261–266 (2018). https://doi.org/10.1038/s41390-018-0062-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0062-4