Abstract
The pathobiology and aggressiveness of the triple negative breast cancer (TNBC) are influenced by genes that are preferentially expressed in TNBC cells. However, the nature of such genes with the role in invasiveness of TNBC cells is not fully understood. Here, we identified FAM171A1, member (A1) of the family with sequence similarity 171, as an overexpressed candidate gene in TNBC cells and tumors as compared to estrogen receptor-alpha (ERα) positive breast cancer. We found that the expression of FAM171A1 correlates well with the loss of ERα as well as its newly identified target miR590-5p in TNBC but not in ERα-positive cells. In addition, we report that ERα regulates FAM171A1 expression through a mechanism which involves ERα stimulation of miR590-5p expression via binding to its promoter, and in-turn, miR590-5p suppression of FAM171A1 expression. Further, we found that the levels of FAM171A1 correlate well with cancer cell aggressiveness as depletion or overexpression of FAM171A1 confers reduced or increased ability of TNBC cells to form mammospheres, respectively in accordance with the previous report of increased mammosphere formation potential of metastatic cells. In brief, results presented here have demonstrated that ERα regulation of FAM171A1 expression via miR590-5p explains the molecular basis of the noticed reduced levels of FAM171A1 in ER-positive breast cancer cells and that FAM171A1 is a preferably TNBC- overexpressed gene. Further, the noted loss of ERα–miR590-5p axis may upregulate the expression of FAM171A1 and consequently, resulting aggressiveness of TNBC cells. These findings suggest that FAM171A1 might represent a potentially novel therapeutic target for TNBC tumors.
Similar content being viewed by others
Introduction
Breast cancer is the most common form of cancer in women1. Despite significant advances in our ability to detect and treat breast cancer, it remains a leading cause of death in women with cancer, and the incidence of breast cancer continues to rise in many parts of the world. Among breast cancers, triple-negative breast cancer (TNBC) sub-type is one of the aggressive breast cancers as TNBC cells lack all three currently targetable molecules such as estrogen receptor, progesterone receptor, and HER2.
The family with sequence similarity 171, member A1 protein (FAM171A1) is a glycoprotein composed of 890 amino acids with a molecular weight of 97,854 Da. It is a single-pass type 1 membrane protein, also called “Astroprincin”. Previous work suggests that FAM171A1 may be associated with chemoresistance of cancer cells2,3. Data-mining studies suggest that FAM171A1 interacts with FAM171B, PCDHGB1, TNFRSF17, TMEM, CTDSPL, and NTRK14,5,6,7, many of which have been implicated in various cancers8,9,10,11,12,13. Emerging reports suggest that FAM171A1 might be overexpressed in TNBC tumors14,15,16. However, the nature of the upstream regulation of FAM171A1 and biological significance of FAM171A1 in breast cancer remains poorly understood, and this is being addressed in the present study.
miRNAs play a pivotal role in human cancer and act as key regulators during tumor initiation, proliferation, and metastasis and thereby thought to be important as diagnostic, predictive, and prognostic biomarkers. Furthermore, the expression profile of specific miRNAs differs between various subtypes of breast cancers. These subtle differences could be possibly attributed due to the differential signaling cascade initiating from estrogen receptor-alpha (ERα), PGR, and Her2. In this context, miR590-5p is reported to be high in ERα-positive MCF-7 cells as compared to ERα-negative MDA-MB231 cells17, while another report found no change in the levels of miR590-5p (also known as OncomiR, anti-anti-OncomiR) between ER+ and ER− breast cancer cells18. A previous report shows that the miR590-5p containing genomic region, along with its promoter, resides within the EIF4H gene19, is an intergenic miRNA20. Further, miR590-5p has been shown to be modified by ERα in MCF-7 cells21,22, through a poorly understood mechanism.
Here we identify FAM171A1 as a preferentially expressed gene in basal-type breast tumors, and its levels closely correlate with the aggressiveness of breast cancer cells. Additionally, we explored the role of ERα and its newly identified target here, the miR590-5p, in the regulation of FAM171A1 expression in ERα+ breast cancer and TNBC cells. We present evidence to demonstrate that overexpression of FAM171A1 promotes the expression of epithelial-to-mesenchymal (EMT) markers as well as supports the ability of MCF-7 and T47D cancer cells to grow in an anchorage-independent manner and form mammospheres; levels of FAM171A1 inversely correlate with the status of ERα; and ERα regulates the expression of FAM171A1 via stimulating the expression of miR590-5p which, in-turn, targets FAM171A1. In brief, the present study reveals a new molecular component of breast cancer aggressiveness and raises the possibility of targeting FAM171A1 in developing novel TNBC-directed future therapeutic approaches.
Results
FAM171A1 overexpression in TNBC breast cancer cell lines and tissues
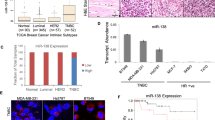
Microarray expression profiles of breast cancer cell lines and breast tumor sub-types such as luminal and basal tumor tissues have been published recently23. We analyzed these data sets to identify the nature of unique genes that might be closely overexpressed in TNBC cells and TNBC breast tumors (Supplementary Fig. 1A). This strategy led us to a single gene, known as FAM171A1—which is widely expressed in the basal-like breast cancer cell lines and tissue samples. Multiple datasets for post-translational modifications predicted that FAM171A1 might be a glycoprotein, and accordingly, we noticed an expected shift in the mobility of FAM171A1 protein upon exposing MDA-MB-231 cells with tunicamycin, which targets glycosylated proteins (Supplementary Fig. 1B). Next, we screened the status of FAM171A1 in a panel of TNBC and non-TNBC breast cancer cell lines. We found high levels of FAM171A1 in the basal-type TNBC cell lines such as MDA-MB-231, SUM149, SUM159, BT549, HCC1937, and Hs-578T as compared to non-TNBC breast cancer cell lines such as MCF-7, T47D, ZR-75, and SKBR3 as well as normal mammary epithelial cell line, HMEC (Fig. 1a–c, Supplementary 4A, B). Consistent with these observations, levels of FAM171A1 were higher in TNBC tumors as compared to non-TNBC tumors as analyzed by Oncomine database (Fig. 1d). Kaplan–Meier survival curve analyses of breast cancer patients by the PrognoScan, the Survival Express, and the KM plotter revealed an increased expression of FAM171A1, and that its levels correlate well with an overall poor survival of the breast cancer patients (Fig. 1e and Supplementary Fig. 1C). However, Kaplan–Meier survival analyses of miR590-5p (a suspected modifier of FAM171A1, this study) show that high expression of miR590-5p may lead to better overall survival rates of breast cancer patients, highlighting its significance in breast cancer biology in physiologically relevant setting (Fig. 1f). In brief, these findings suggest that FAM171A1 might be preferentially involved in the pathobiology of TNBC tumors.
a Western blot analysis for FAM171A1 in a panel of cell lines. b, c qRT-PCR analysis of FAM171A1 and ERα in breast cancer cell lines. Unpaired two-tailed t-test; *p = 0.033 for FAM171A1; **p = 0.002 for ERα. d Boxplots showing mRNA expression of FAM171A1 among TNBC and non-TNBC tumor samples in three Oncomine datasets: TCGA, Curtis, and Kao’s breast; datasets ordered by overexpression Gene Rank-top 10%, a threshold at p value = 1E−4. e Kaplan–Meier survival curve analyses of FAM171A1 in patients with breast cancer in GSE3143 (n = 158) and Breast Invasive Carcinoma TCGA (n = 502), datasets stratified based on high (red) and low (blue) in case of GSE3143 dataset and high (red) and low (green) in case of Breast Invasive Carcinoma TCGA; and *p < 0.002, log-rank test. f Overall survival curve of breast cancer patients with hsa-miR590-5p expression in METABRIC (n = 1262) and GSE19783 (n = 101), datasets stratified based on high (black) and low (red); log-rank test. All experiments were performed in biological replicates and three technical replicates with similar results
ERα downregulates FAM171A1 expression
Next, we set to understand the basis of noticed reduced expression of FAM171A1 in ERα-positive breast cancer cells as compared to ERα-negative breast cancer cells. We tested the hypothesis that ERα might be responsible for the noted reduced expression of FAM171A1 in ERα-positive breast cancer cells. If this is true, then the loss of ERα in TNBC cells might possibly explain the noticed increased expression of FAM171A1 in TNBC cells and tumors. To experimentally test this notion, we first analyzed the published breast tumor datasets using cBioPortal tools. We found that indeed, the expression of FAM171A1 and ESR1 (ERα) mRNAs are mutually exclusive (Fig. 2a, b). We then performed extensive mining of the Oncomine data resource and found that in general, FAM171A1 expression negatively correlates with the status of ESR1 (ERα) (Fig. 2c). As ERα (ESR1) expression strongly parallels to the PR (PGR; Progesterone receptor), we next tried to assess the correlation between the ESR1, PGR, and FAM171A1 in published cell line datasets as well as in other cancer datasets. This analysis revealed that in general, ESR1 expression exhibits a negative correlation with FAM171A1 in breast cancer datasets, and a similar trend was also observed for PGR in respective cell lines as well as carcinoma databases other than breast cancer datasets (Supplementary Fig. 2A, B, C, and D). These observations raised the possibility of reciprocal regulation of FAM171A1 by ESR1 or vice-versa. To address this, we knocked down the levels of endogenous FAM171A1 in MDA-MB-231 cells and analyzed the expression levels of ESR1. We did not observe any effect of FAM171A1 depletion on the levels of ERα (Supplementary Fig. 1D).
a Expression of FAM171A1 and ESR1 (ERα) in METABRIC (n = 2051) and TCGA (n = 1084) databases analyzed by cBioPortal tool. b Scatter plot analysis of ESR1 (ERα) and FAM171A1 mRNA expression using cBioPortal; Pearson correlation: 0.394; Spearman: −0.578. c Boxplots showing the mRNA expression of FAM171A1 in three Oncomine datasets stratified based on ERα positive and ERα negative status; TCGA (n = 593), Curtis (n = 2136), and Hatzis (n = 508); datasets ordered by overexpression Gene Rank; top 10%, a threshold at p-value = 1E−4. d, e Western blot analysis showing the effect of overexpression of ERα on FAM171A1 in MDAMB231 cells cultured in both FBS containing DMEM media and CTS containing phenol-red free DMEM. Experiments (d, e) were performed three times with similar results
Next, we analyzed the regulation of FAM171A1 by modulating the status of ERα. We noticed that the ectopic expression of ERα in MDA-MB-231 cells leads to significant repression of FAM171A1 (Fig. 2d and Supplementary Fig. 1E). To understand the role of weak estrogenic phenol red in modulating the expression of FAM171A1, we examined the expression of FAM171A1 in MDA-MB-231 cells cultured in phenol-red free culture medium supplemented with 5% charcoal-stripped serum and examined the impact of ERα expression upon the status of FAM171A1. As suspected, ERα overexpression in the absence of estradiol leads to a reduction in the level of FAM171A1 protein and transcript (Fig. 2e and Supplementary Fig. 1F), suggesting a contribution of experimental culture conditions in residual levels of FAM171A1. Similar results were noticed in SUM149 and SUM159 cell lines (TNBCs) (Supplementary Fig. 4C, D and E). These findings are consistent with the notion that unliganded ERα alone represses multiple gene signatures as supported by the published report24,25.
Regulation of FAM171A1-UTR activity by miR590-5p
Having shown a repressive role of ERα on FAM171A1 gene, we next analyzed the mechanism by which ERα regulates the level of FAM171A1. Using sequence retrieval tool from EPD database26, first we extracted the promoter sequence from −2500 bp to 100 bp relative to transcription start site (TSS) and screened for the predicted transcription factor binding motifs within the FAM171A1 promoter using ALGGEN-PROMO database while keeping dissimilarity margin less or equal than 0%. We found that FAM171A1 promoter contains one ERE consensus sequence (GGTCAGACTGACT) at −1261 bp. In addition, we also used the JASPAR database, setting up the p-value to 0.0001, and found only two ERE sites at the locations −15 bp and −1525 bp. However, ChIP experiments using anti-ERα-Ab failed to exhibit any recruitment of ERα onto the promoter of FAM171A1 as opposed to the recruitment of ERα onto the promoters of PS2 and Cyclin D1, two ERα responsible genes (Fig. 3a).
a Chromatin Immunoprecipitation analysis showing lack of ERα recruitment onto the FAM171A1 promoter; pS2 and Cyclin D as positive controls. b Diagram showing three seed sequences at designated locations within the 3ʹ-UTR of the FAM171A1 gene. c Endogenous expression of miR590-5p in breast cancer cell lines as analyzed by qRT-PCR using TaqMan chemistry; ***p < 0.0003. d qRT-PCR showing the induction of preMIR590 expression upon ERα transfection in MDAMB231 cell line. The data were normalized to U6 snRNA. e Schematic diagram showing the putative ERα-binding region in the MIR590 promoter. Exon in light gray color, ERE and STAT5 binding sites in black color. f CHIP analysis of ERα binding onto the MIR590 promoter in MCF-7 and T47D cells. SG6K and DNAMT3A primers were used as negative controls. g pSi-CHECK2 and pRIP vector constructs showing the regions for cloning of 3ʹ-UTR region and MIR590 gene, respectively. h Effect of ERα cotransfection upon miR-590 promoter-luc reporter activity; *p-value < 0.05 (Student’s t-test). i Effect of miR-590-pRIP expression on the 3ʹ-UTR-pSi-CHECK2-luc activity; *p-value < 0.05 (Student’s t-test). Experiments (a, c, f, h, i) were performed in three biological replicates and d was performed two times each time with three technical replicates
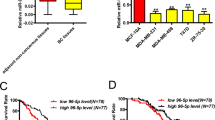
Since we observed repression of FAM171A1 mRNA upon ERα transfection in MDA-MB-231 cells both in the case of activated and unliganded ERα (presumably, due to the ligand-independent action of ERα), we next searched for another possible mechanism of FAM171A1 regulation by ERα. We examined for the status of specific miRNAs in the context of ERα-positive cancer cells. We identified miR590-5p as a candidate gene with three seed sequences within a single 3ʹ-UTR sequence of FAM171A1 using “microrna.org” database (Fig. 3b). miR590-5p has been shown to be well expressed in MCF-7 cells compared to MDA-MB-231 cells17,21. To validate these results, we determined the status of endogenous miR590-5p in breast cancer cells. Our result shows that the levels of miR590-5p are elevated in ERα-positive breast cancer cells than in ERα-negative breast cancer cells (Fig. 3c).
ERα regulation of FAM171A1 via MIR590 expression
Next, we examined the effect of ERα on the levels of endogenous miR590-5p in MDA-MB-231 cells and found that ERα overexpression upregulates the level of pre-miR590 in breast cancer cells (Fig. 3d). To investigate whether miR590-5p targets the 3ʹ-UTR region of FAM171A1 upon modulation of ERα. We used miRStart database and noticed that miR590-5p promoter contains a single ERα binding site at the location −1050 bp upstream of TSS (−4780 from the precursor miR590) (Fig. 3e). We found that ERα gets recruited onto the miR590-5p promoter in ChIP assays, as opposed to the promoters of SG6K and DNAMT3a—two ERα unresponsive genes (Fig. 3f). To examine a direct role of ERα in the expression of miR590-5p, we cloned miR590-5p promoter and demonstrated that ERα stimulates miR590-5p transcription (Fig. 3h).
To establish a direct role of miR590-5p in the regulation of FAM171A1 expression, we cloned the 3ʹ-UTR region of FAM171A1 into a psi-CHECK2 vector while miR590-5p was cloned into a pRIP vector (Fig. 3g). We found that miR590-5p binds and suppresses the transcription from FAM171A1–3ʹ-UTR reporter system (Fig. 3i). Together these findings suggest that ERα stimulates miR590-5p, which, in-turn, represses FAM171A1 expression, and that the noticed increased expression of FAM171A1 might be associated with low levels of miR590-5p in ERα-negative TNBC breast cancer cells.
FAM171A1 regulation of TNBC biology
To understand the functional significance of the noticed increased expression of FAM171A1 in breast cancer biology, we evaluated the effect of depleting endogenous FAM171A1 in MDA-MB-231 cells on its invasiveness, anchorage-independent growth, and ability to form mammospheres. We found that depletion of FAM171A1 leads to a reduced invasiveness of MDA-MB-231 cells as supported by reduced expression of EMT markers such Snail, Slug, E-Cadherin as well as decreased invasion as assessed by invasion assay in MDA-MB-231 cells suggesting a role of FAM171A1 in supporting cell invasiveness of TNBC cell (Supplementary Fig. 3A and 3B). In addition, FAM171A1 knockdown in MDA-MB-231 cells is also accompanied by a reduction in the colony-forming ability of MDA-MB-231 cells (Fig. 4a, b, and c). Interestingly, we also noticed that FAM171A1 knockdown in MDA-MB-231 leads to a reduced number and size of mammospheres (Fig. 4d and e) as well as reduced expression of stem cell markers such as Oct4 and Sox2 (Supplementary Fig. 3C). Similar to MDA-MB-231 cells, we also found that stable knockdown of FAM171A1 in SUM149 TNBC cells is also accompanied by reduction in the levels of EMT markers such as Snail, Slug, and Zeb1 assessed by Western immunoblotting (Supplementary Fig. 3D) and reduced ability of cells to form anchorage-independent colonies as well as mammospheres (Fig. 4f, g, h, i, and j). Further, to evaluate the association of FAM171A1 with stemness, we utilized Gene Set Enrichment Analysis (GSEA) of GSE36693 dataset in order to see any possible overlap between profiles with high expression of FAM171A1 and stem cell gene signatures. Analysis revealed that high expression of FAM171A1 correlates with upregulated stem cell gene signatures and therefore, may be involved in the regulation of cancer stem cell functions (Supplementary Fig. 5A). Additionally we also observed activated gene signatures for TGFβ/Smad and Wnt/β-catenin pathways27,28 suggesting, these stem cell-associated pathways could possibly be linked with FAM171A1 (Supplementary Fig. 5B).
a qRT-PCR and Western blot analyses of FAM171A1 mRNA and protein upon silencing in MDA-MB-231. b, c Anchorage-independent colony formation assay showing a decreased proliferation of MDA-MB231 cells upon FAM171A1 knockdown. d, e Representative image of the tumorspheres formed by MDA-MB-231 vs silenced MDA-MB-231 cells; scale bar: 100 µm; quantification on the top. f Status of FAM171A1 mRNA and protein in SUM149-vector and SUM149-shFAM171A1 stable cell line. g, h Anchorage-independent colony formation assay showing a decreased proliferation of SUM149 cells upon stable knockdown of FAM171A1; quantification on the top. i, j Representative image of the tumorspheres formed by SUM149 vector vs. SUM149-shFAM171A1 stable cells; scale bar: 25 µm, quantification on top; ***p-value < 0.0003; **p-value < 0.005; *p-value < 0.05; unpaired two-tailed t-test. All experiments were performed three times and with three technical replicates where needed
To further examine the role of FAM171A1 in supporting the stemness of breast cancer cells, we next generated stable clones from MCF-7 and T47D breast cancer cells which otherwise express no or low levels of FAM171A1 (Fig. 5a, d). We found that FAM171A1 overexpression in MCF-7 and T47D cells leads to increased mammosphere-forming capability (Fig. 5b, c, e, f and Supplementary Fig. 3E) as well as EMT markers such as Snail, Slug, Zeb1, and N-Cad in case of MCF-7/FAM171A1 cells whereas Snail, Slug, Zeb1, Vimentin, and Claudin-1 in case of T47D/FAM171A1 stable cells (Supplementary Fig. 3F, 3G, and 3H). These differences could be attributed to the differences in the cell line’s gene expressions. Ectopic expression of FAM171A1 also increased stem cell markers such as Oct4, Sox2, and Nanog as observed in MCF-7/FAM171A1 as well as T47D/FAM171A1 stable clones (Supplementary Fig. 3I and 3J) as compared to the control cells. These results suggest that one of the roles of FAM171A1 in breast cancer cells might be to support their stemness, and in turn, aggressiveness29.
a Western blot analysis of FAM171A1 expression in MCF-7 stably overexpressing FAM171A1. b, c Representative images of tumorspheres formed by the indicated cells (below); scale bar: 50 μm, quantification on top; **p-value = <0.005, unpaired two-tailed t-test. d Western blot analysis of FAM171A1 in T47D stably expressing FAM171A1. e, f Representative images of tumorspheres formed by the indicated cells (below); scale bar: 50 μm, quantification on top; **p-value < 0.005, unpaired two-tailed t-test. g Reduction in the levels of FAM171A1 expression upon miR-590-5p mimic treatment in MDA-MB-231 cells. All experiments were performed three times with similar results
We next examined the effect of modulating the endogenous levels of FAM171A1 with exogenously supplied synthetic miR590-5p mimic/LNA (locked nucleic acid) on the ability of cells to form mammosphere. Result in Fig. 5g illustrates that miR590-5p mimic treatment in SUM149 cells reduced the expression of the FAM171A1 protein (Fig. 5g). Finally, we validated that miR590-5p mimic inhibited the ability of MDA-MB-231 and SUM149 cells to form anchorage-independent colonies and mammosphere, whereas miR590-5p LNA closely increases the ability to form anchorage-independent colonies and mammosphere (Fig. 6a, b, c, and d). Altogether, these findings support the notion that miR590-5p is an endogenous regulator of FAM171A1 expression and that increased expression of FAM171A1 in TNBC cells contributes to invasive phenotypes.
a, b Effect of miR590-5p mimic and LNA treatments on the growth of MDA-MB-231 and SUM149 as assessed by colony formation assay; quantification on top. c, d Quantification of the tumorspheres formed upon miR-590-5p mimic and LNA treatments in MDA-MB-231 and SUM149 cells; *p-value < 0.05, one-way ANOVA; **p-value < 0.005. e Working model showing the summary of the regulation of the ERα–miR590-5p–FAM171A1 axis in breast cancer cells. Experiments were performed in two biological replicates each with two technical replicates with similar results
Discussion
In summary here, we demonstrate FAM171A1 as a candidate gene overexpressed in TNBC cell lines and TNBC tumors as compared to ERα-positive breast cancer cells. The levels of FAM171A1 are albeit in case of immortalized mammary epithelial cells when compared with ERα-positive or ERα-negative cell lines. High expression of FAM171A1 is associated with overall poor survival of the breast cancer patients, in contrast to miR590-5p, whose elevated expression may lead to better overall survival rates as assessed by the KM plots suggesting an inverse correlation between clinical significance of these two genes in the survival of breast cancer patients. In silico and data-mining analyses suggest that FAM171A1 has been predicted to interact with FAM171B, PCDHGB1, TNFRSF17, TMEM, CTDSPL, and NTRK1, many of which have been already implicated in various cancers suggesting most likely its possible role in the tumorigenesis. ERα, as well as its target PGR (PR), is an established biomarker in breast cancer patients. However, we observed a strong correlation between FAM171A1 and ESR1 (ERα) but not with PGR. This provided the basis of exploring the underlying relationship between ERα and FAM171A1 utilizing bioinformatics datasets, such as Oncomine, TCGA, cBioPortal, and in previously published raw microarray data. Here for the first time, we provide the molecular basis of the noted loss of FAM171A1 that is highly expressed in ERα-deficient breast cancer cells.
During the course of this study, we identified miR590-5p as an ERα target, the expression of which is elevated in ERα-positive, non-TNBC cells such as MCF-7, T47D, and ZR-75 cells when compared to ERα-negative (TNBC) cell lines such as MCF10A, BT549, MDA-MB-231, SUM149, SUM159, HCC1937, and Hs-578T cells. Further, using the ChIP coupled with Luciferase assays, we found that ERα induces the expression of miR590-5p by binding to its promoter and positively regulates its expression.
The underlying mechanism of the noticed upregulation of FAM171A1 in TNBC cells includes reduced levels of miR590-5p in TNBC cells, which otherwise targets FAM171A1 in ERα-positive breast cancer cells, and thus the loss of miR590-5p regulates FAM171A1 in TNBC cells. Mechanistically, we show that miR590-5p is an ERα-responsive gene and that ERα stimulates the expression of miR590-5p, which, in turn, downregulates FAM171A1 in ERα-positive breast cancer cells. These findings suggest that absence of ERα, as well as its target miR590-5p in TNBC cells, contribute, at least, in part, to the observed increased expression of FAM171A1 in breast cancer cells. Functional studies showed that elevated expression of FAM171A1 in breast cancer cells contributes to the invasiveness and stemness of breast cancer cells.
GSEA reveals an overlap between the high level of FAM171A1, upregulated stem cell gene signatures as well as activated gene signatures for TGFβ/Smad and Wnt/β-catenin pathways, suggesting a possible involvement of FAM171A1 in the modulation of cancer stem cells. We propose that the noted absence of ERα and its target miR590-5p in TNBC cell lines might be one of the contributing factor for driving the observed elevated endogenous expression of FAM171A1. Future studies are needed to understand the nature of signaling pathways under the influence of FAM171A1 and their targets responsible for the observed biological functions of FAM171A1. In brief, results presented here suggest that the FAM171A1 is an important TNBC-specific gene as its level profoundly modulates the aggressiveness of breast cancer cells. To the best of our knowledge, this is the first report showing the mutual exclusivity between the levels of FAM171A1 and ERα, and underlying mechanism, at least, in part, wherein ERα influences the expression of FAM171A1 via miR590-5p.
Materials and methods
Materials
Breast cancer cell lines MCF-7, T47D, MDA-MB-231, SKBR3, BT549, ZR-75, and Hs-578T were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 µg/ml streptomycin. HCC1937 (breast cancer cell line) was cultured and maintained in RPMI medium whereas MCF10A, SUM 149, and SUM 159 (breast cancer cell lines) were cultured and maintained in Mammary Epithelial Cell Growth medium (MEGMTM from Lonza) with fibroblast growth factor, heparin, bovine pituitary extract, hEGF, insulin, hydrocortisone, and gentamicin/amphotericin. Total RNA was isolated from all the cell lines using TRIzol reagent (Invitrogen), and MIRVANA kit was used for the isolation of miRNAs according to the manufacturer’s instructions. TaqMan® MicroRNA Assays for hsa-miR-590-5p, hsa-miR-590–3p, mirVana® miRNA mimic, mirVana® miRNA inhibitor, and mirVana® miRNA negative control were used from Invitrogen company. Simple ChIP® Kit (Cat #9003) was obtained from CST. Antibodies against FAM171A1 (GTX120226), HER2 (CST 2908), ERα (sc-8005), HSC70 (sc-7298), and β-actin(C4) (sc-47778) were purchased from respective companies as indicated by the alphabets on the catalog numbers. Snail (C15D3), Slug (C19G7), E-cadherin (24E10), N-cadherin (D4R1H), Vimentin (D21H3), Zeb1 (D80D3), and Claudin-1 (D5H1D) antibodies were used from Cell Signaling Technology. FAM171A1 siRNA (sc-90617) and ERα siRNA (sc-29305) were ordered from Santa Cruz Biotechnology (Santa Cruz, CA, USA). pEGFP-C1-ERα (28230) plasmid was obtained from Addgene. FAM171A1 shRNA (human) was procured from Sigma (TRCN0000264229). Primers used in the study are enlisted in Table 1.
Western blot analysis
Cells were harvested and lysed in 50–100 µl lysis buffer. Lysates were clarified by centrifugation at 14,000 rpm for 15 min. A total of 50 μg protein was denatured, processed, and loaded for SDS-PAGE, which was performed using 8% gels. The proteins were electrophoretically transferred to PVDF membranes (AmershamTM HybondTM P 0.23 µm PVDF). The blots were then blocked with 3% BSA in Tris-buffered saline (TBS, pH 7.6) for 1 h and then incubated with primary antibodies in 3% BSA in TBST overnight. After washing with TBST (TBS with 0.1% Tween-20), the membranes were incubated with HRP conjugated secondary antibodies for 1 h at room temperature and were visualized by enhanced chemiluminescence (MILLIPORE ImmobilonTM Western HRP Substrate Luminol Reagent) and developed on X-ray film (Carestream, Colorado, USA).
Knockdown/overexpression studies
MDA-MB-231 cells were seeded in 6-well plates 1 day before transfection to reach 70% confluency by the next day. For overexpression studies, cells were transfected with 1 µg plasmids, and for knockdown studies, 120 pmol of FAM171A1 siRNA was standardized and used for the efficient knockdown. Plasmid transfections and shRNA transfection was done using Lipofectamine® LTX and PlusTM Reagent (Invitrogen, Carlsbad, CA, USA) and Lipofectamine 3000 reagent whereas siRNA transfection was done using Lipofectamine RNAi Max (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s protocol.
Quantitative reverse transcription-PCR
Total cellular RNA was extracted using TRIzol, and cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). cDNAs were used for quantitative reverse transcription-PCR (qRT-PCR) analysis using SYBR-Green Master PCR mix (Roche) on a Step One Plus Real-Time PCR system (Applied Biosystems, USA). GAPDH was used as a normalizing control in the case of gene expression studies whereas RNU48 was used as an endogenous control in case of miRNAs expression study. For individual quantitation of miRNA, RNA isolation was performed using mirVana™ miRNA Isolation Kit (Cat. No. AM1560; Thermo Fisher Scientific). About 50 ng of total RNA was converted to cDNA using TaqMan miRNA Reverse Transcription kit (ABI) according to the manufacturer’s guidelines, which was then analyzed using TaqMan probes miR-590-5p, miR-590-3p, and RNU44/48 obtained from Invitrogen.
Bioinformatics studies
To examine the binding of ERα to the FAM171A1 promoter, we analyzed 2.5-kb promoter sequence upstream of TSS for the predicted transcription factor binding sites using the transcription factor binding tools—JASPAR, ALGGEN PROMO, and EPD databases. Promoter motifs such as TATA box, CCAAT box, GC box as well as initiator motif were analyzed by Eukaryotic Promoter Database. FAM171A1 gene is transcribed from the minus strand. Since no validated transcription factors are reported for FAM171A1 gene so far, we tried to predict the transcription factors bound to its promoter based on the least dissimilarity score of “0” in case of ALGGEN PROMO whereas p-value 0.0001 for EPD and JASPAR. Conventionally, we set out to find the presence of ERE (ER response element, GGTCAnnnTGACC) sequence in the promoter region. We noticed a perfect ERE consensus sequence in the promoter of FAM171A1 within 2 kb region. This raised the possibility that ERα could bind to the FAM171A1 promoter either directly or through a co-regulatory complex. For the ERα binding site within the MIR590 promoter, we analyzed ~2.5-kb upstream sequence from TSS for MIR590 gene.
Chromatin immunoprecipitation
MCF-7 and T47D cells were grown in three 100 mm culture dishes. After 80% confluency was reached, the cells were fixed with formaldehyde and CHIP protocol was performed using SimpleChiPTM Enzymatic Chromatin IP Kit (Agarose Beads) from Cell Signaling Technology according to their protocol. For sonication, 14 cycles were standardized for the required fragment sizes with 30 s ON and 30 s OFF. The ChIP products were analyzed by PCR for both FAM171A1 as well as miRNA-590 promoter’s binding using the primers listed above in the text.
Colony formation assay
One-thousand cells were seeded in each well of 6-well plates for MDA-MB-231 and 500 for SUM149. The plates were kept back in the CO2 incubator for nearly about 10–12 days. The media were changed every 3 days. On the last day, the media were aspirated, and the wells were washed with 1× PBS very carefully. Colonies were fixed with 10% neutral formalin (1 ml formalin for each well of 6-well plate) for nearly half an hour. Then the wells were again rinsed with 1× PBS carefully. Colonies were then stained with 1 ml of 0.5% crystal violet stain, incubated for 30 min, and wells were destained under running tap water very carefully.
Mammosphere formation assay
Cells were grown in T-25 flask up to 70–80% confluency, trypsinized and resuspended in MEBM media containing 10% FBS and reseeded in 96-well low attachment surface plates containing 200 cells in each well having 200 µl of MEBM media. Plates were then incubated in the CO2 incubator for 10–12 days for the formation of spheres. After 10–12 days, both the number and the size of the spheres were observed and counted.
Invasion assay
The protocol followed was according to manufacturer’s guidelines. Briefly, a plate was taken out from −20 °C and kept at room temperature for nearly 10–15 min; 500 µl of warm (37 °C) DMEM was added to each apical chamber and kept for 2 h in a 37 °C incubator with 5% CO2. After rehydration, the medium was carefully removed from each apical chamber without disturbing the layer of BD Matrigel™ Matrix on the membrane. Cell suspensions were made in serum-free DMEM and 2 × 104 cells were seeded on to the top of each apical chamber. Finally, the total volume of the cell suspensions was made up to 500 µl by adding additional serum-free DMEM to the apical chamber if required. Lower chambers were filled with 750 µl of 5% FBS serum containing DMEM as a chemo-attractant. BD BioCoat™ Tumor Invasion System and the uncoated BD Falcon FluoroBlok™ 24-Multiwell Insert System were then incubated for 20 h at 37 °C, 5% CO2. Following incubation, the medium was removed from apical as well as the lower chamber, replaced with Hoechst dye (2 µg/ml) containing phenol red-free DMEM in the lower chamber and incubated for 15 min. Then, the dye-containing medium was aspirated out and replaced with 1× PBS for washing the lower side of the apical chamber carefully just by dipping. Invaded cells were imaged and counted by nuclear stain.
miR-590 promoter luciferase assay
MDA-MB-231 cells were transfected with 200 ng of pGL3 vector cloned with the miRNA-590 promoter or pGL3 control; 50 ng of Renilla luciferase vector was transfected, serving as a transfection control. The cells were co-transfected with pcDNA3.1 and ERα vectors where necessary. After 48 h, the cells were lysed using Glo Lysis Buffer (Promega), and the lysate was clarified by centrifuging at 14,000 rpm for 15 min. Firefly and Renilla luciferase activities were measured using RenillaGlo and SteadyGlo reagents (Steady-Glo-Luciferase Assay System and Renilla-Glo-Luciferase Assay System from Promega) according to manufacturer's guidelines. Firefly readings were normalized to renilla luciferase readings to generate relative luciferase activity.
3ʹ-UTR luciferase assay
MCF-7 cells were seeded into 24-well plates to 80% confluency. Next day, the cells were transfected using Lipofectamine LTX plus with 200 ng psiCHECK2 vector encoding the entire 3′-UTR region of FAM171A1 gene fused downstream of the renilla luciferase gene and the firefly luciferase gene as a reporter along with 200 ng pRIP plasmid constructs encoding miR-590 or pRIP control plasmid vector where required and the firefly luciferase gene as a reporter. After 48 h incubation, the cells were assayed with the Dual-Luciferase® Reporter Assay System (Promega) to measure the renilla luciferase and firefly luciferase activity, which served as a transfection control. In brief, the 24-well plate was taken out, and the cells were washed once with 1× PBS. For cell lysis, 100 µl of 1× Passive Lysis Buffer was added to each well and incubated for 15 min over a shaker at maximum speed for efficient lysis. Cells were then scraped/pipetted up–down. Lysed cells were collected in microfuge tubes and centrifuged for 15 min at 14,000 rpm. The supernatant was collected (~100 µl) and stored at −80 °C. Luciferase readings were taken using a Luminometer (TD20/20, Promega) system according to manufacturer’s guidelines.
miR590-5p LNA/mimic experiment
About 2.5 × 105 cells were seeded in 6-well plates. For transfection, 30 nM miR590-5p mimic, as well as the inhibitor, were used. RNAimax from Invitrogen was used for transfection according to the manufacturer’s protocol. Post 48 h, the cells were trypsinized and seeded for colony formation as well as sphere formation assay.
Statistics
Where necessary, statistical data were analyzed using GraphPad Prism to generate SE values and to determine the level of significance using the Student’s t-test (one-tailed or two-tailed, as appropriate) and one-way ANOVA; *p-value < 0.05; **p-value 0.005, ***p-value 0.0001 were considered to indicate significance. Data are reported as mean ± SEM.
References
De Santis. et al. Breast Cancer Statistics, 2013. CA Cancer J. Clin. 64, 52–62 (2014).
Liu, L. et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 69, 6871–6878 (2009).
Calcagno, A. M. et al. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J. Natl Cancer Inst. 102, 1637–1652 (2010).
Huttlin, E. L. et al. Architecture of the human interactome defines protein communities and disease networks. Nature 545, 505–509 (2017).
Gupta, G. D. et al. A dynamic protein interaction landscape of the human centrosome-cilium interface. Cell 163, 1484–1499 (2015).
St-Denis, N. et al. Phenotypic and interaction profiling of the human phosphatases identifies diverse mitotic regulators. Cell Rep. 17, 2488–2501 (2016).
Emdal, K. B. et al. Temporal proteomics of NGF-TrkA signaling identifies an inhibitory role for the E3 ligase Cbl-b in neuroblastoma cell differentiation. Sci. Signal. 8, ra40 (2015).
Pelekanou, V. et al. BCMA (TNFRSF17) induces APRIL and BAFF mediated breast cancer cell stemness. Front. Oncol 8, 301 (2018). https://doi.org/10.3389/fonc.2018.00301.
Chae, S.-C. et al. The haplotypes of TNFRSF17 polymorphisms are associated with colon cancer in a Korean population. Int. J. Colorectal Dis. 27, 701–707 (2012).
Oktay, M. H. & Jones, J. G. TMEM: a novel breast cancer dissemination marker for the assessment of metastatic risk. Biomark. Med. 9, 81–84 (2015).
Chen, D. et al. Genome-wide CNV study and functional evaluation identified CTDSPL as tumour suppressor gene for cervical cancer. bioRxiv. 353391 (2018). https://doi.org/10.1101/353391.
Youssef, G., Gillett, C., Agbaje, O., Crompton, T. & Montano, X. Phosphorylation of NTRK1 at Y674/Y675 induced by TP53-dependent repression of PTPN6 expression: a potential novel prognostic marker for breast cancer. Mod. Pathol. 27, 361 (2014).
Amatu, A., Sartore-Bianchi, A. & Siena, S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 1, e000023 (2016).
Santuario-Facio, S.K. et al. A new gene expression signature for triple negative breast cancer using frozen fresh tissue before neoadjuvant chemotherapy. Mol. Med 23, 101–111 (2017).
Stayrook, K. R. et al. TGFbeta-Mediated induction of SphK1 as a potential determinant in human MDA-MB-231 breast cancer cell bone metastasis. Bonekey Rep. 4, 719 (2015).
Al-Ejeh, F. et al. Meta-analysis of the global gene expression profile of triple-negative breast cancer identifies genes for the prognostication and treatment of aggressive breast cancer. Oncogenesis 3, e100 (2014).
Miranda, P. J., Vimalraj, S. & Selvamurugan, N. A feedback expression of microRNA-590 and activating transcription factor-3 in human breast cancer cells. Int. J. Biol. Macromol. 72, 145–150 (2015).
Sheikholeslami, A., Nabiuni, M. & Arefian, E. Suppressing the molecular signaling pathways involved in inflammation and cancer in breast cancer cell lines MDA-MB-231 and MCF-7 by miR-590. Tumour Biol. 39, 1010428317697570 (2017).
Tajima, K. et al. SETD1A modulates cell cycle progression through a miRNA network that regulates p53 target genes. Nat. Commun. 6, 8257 (2015).
Monteys, A. M. et al. Structure and activity of putative intronic miRNA promoters. RNA 16, 495–505 (2010).
Bailey, S. T., Westerling, T. & Brown, M. Loss of estrogen-regulated microRNA expression increases HER2 signaling and is prognostic of poor outcome in luminal breast cancer. Cancer Res. 75, 436–445 (2015).
Edvardsson, K. et al. Estrogen receptor beta expression induces changes in the microRNA pool in human colon cancer cells. Carcinogenesis 34, 1431–1441 (2013).
Lee, S. T. et al. Protein tyrosine phosphatase UBASH3B is overexpressed in triple-negative breast cancer and promotes invasion and metastasis. Proc. Natl Acad. Sci. USA 110, 11121–11126 (2013).
Ding, L. et al. Ligand-independent activation of estrogen receptor alpha by XBP-1. Nucleic Acids Res. 31, 5266–5274 (2003).
Caizzi, L. et al. Genome-wide activity of unliganded estrogen receptor-alpha in breast cancer cells. Proc. Natl Acad. Sci. USA 111, 4892–4897 (2014).
Dreos, R., Ambrosini, G., Groux, R., Cavin Perier, R. & Bucher, P. The eukaryotic promoter database in its 30th year: focus on non-vertebrate organisms. Nucleic Acids Res. 45(D1), D51–d5 (2017).
Vermeulen, L. et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12, 468 (2010).
Oshimori, N., Oristian, D. & Fuchs, E. TGF-β promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell 160, 963–976 (2015).
Guttilla, I., et al. Prolonged mammosphere culture of MCF-7 cells induces an EMT and repression of the estrogen receptor by microRNAs. Breast Cancer Res. Treat. 132, 75–85 (2012).
Acknowledgements
We extend our sincere thanks to Dr. Ani V. Das for her kind help in sharing her knowledge for miRNA and 3ʹ-UTR cloning and Deivendran S for his support during this work. We thank Dr. Beena Pillai, from ICGEB, India, for providing us the pRIP vector for microRNA cloning and psi-Check2 vector for 3ʹ-UTR cloning. This work was supported by Department of Biotechnology, Govt. of India, for translational research on TNBC (BT/01/CEIB/01CB/2016). Rahul Sanawar is supported by a Senior Research Fellowship from the University Grants Commission, India (Ref. No: 23/06/2013(i)EU-V).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanawar, R., Mohan Dan, V., Santhoshkumar, T.R. et al. Estrogen receptor-α regulation of microRNA-590 targets FAM171A1—a modifier of breast cancer invasiveness. Oncogenesis 8, 5 (2019). https://doi.org/10.1038/s41389-018-0113-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41389-018-0113-z
This article is cited by
-
Aberrant NSG1 Expression Promotes Esophageal Squamous Cell Carcinoma Cell EMT by the Activation of ERK Signaling Pathway
Digestive Diseases and Sciences (2023)
-
Breast cancer-derived exosomal lncRNA SNHG14 induces normal fibroblast activation to cancer-associated fibroblasts via the EBF1/FAM171A1 axis
Breast Cancer (2023)
-
Gene-associated methylation status of ST14 as a predictor of survival and hormone receptor positivity in breast Cancer
BMC Cancer (2021)
-
Elevated tumor expression of Astroprincin (FAM171A1) is an independent marker of poor prognosis in colon cancer
BMC Gastroenterology (2021)
-
Exploring specific prognostic biomarkers in triple-negative breast cancer
Cell Death & Disease (2019)