Abstract
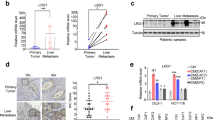
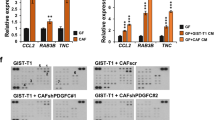
Patients with the mesenchymal subtype colorectal cancer (CRC) have a poor prognosis, in particular patients with stroma-rich tumors and aberrant SMAD4 expression. We hypothesized that interactions between SMAD4-deficient CRC cells and cancer-associated fibroblasts provide a biological explanation. In transwell invasion assays, fibroblasts increased the invasive capacity of SMAD4-deficient HT29 CRC cells, but not isogenic SMAD4-proficient HT29 cells. A TGF-β/BMP-specific array showed BMP2 upregulation by fibroblasts upon stimulation with conditioned medium from SMAD4-deficient CRC cells, while also stimulating their invasion. In a mouse model for experimental liver metastasis, the co-injection of fibroblasts increased metastasis formation of SMAD4-deficient CRC cells (p = 0.02) but not that of SMAD4-proficient CRC cells. Significantly less metastases were seen in mice co-injected with BMP2 knocked-down fibroblasts. Fibroblast BMP2 expression seemed to be regulated by TRAIL, a factor overexpressed in SMAD4-deficient CRC cells. In a cohort of 146 stage III CRC patients, we showed that patients with a combination of high stromal BMP2 expression and the loss of tumor SMAD4 expression had a significantly poorer overall survival (HR 2.88, p = 0.04). Our results suggest the existence of a reciprocal loop in which TRAIL from SMAD4-deficient CRC cells induces BMP2 in fibroblasts, which enhances CRC invasiveness and metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350.
Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. 2015;47:320.
Mesker WE, Junggeburt JM, Szuhai K, de Heer P, Morreau H, Tanke HJ, et al. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol. 2007;29:387–98.
Dekker TJA, van de Velde CJH, van Pelt GW, Kroep JR, Julien JP, Smit VTHBM, et al. Prognostic significance of the tumor-stroma ratio: validation study in node-negative premenopausal breast cancer patients from the EORTC perioperative chemotherapy (POP) trial (10854). Breast Cancer Res Treat. 2013;139:371–9.
Huijbers A, Tollenaar RAEM, v Pelt GW, Zeestraten ECM, Dutton S, McConkey CC, et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: validation in the VICTOR trial. Ann Oncol. 2013;24:179–85.
Wang K, Ma W, Wang J, Yu L, Zhang X, Wang Z, et al. Tumor-stroma ratio is an independent predictor for survival in esophageal squamous cell carcinoma. J Thorac Oncol. 2012;7:1457–61.
Lv Z, Cai X, Weng X, Xiao H, Du C, Cheng J, et al. Tumor–stroma ratio is a prognostic factor for survival in hepatocellular carcinoma patients after liver resection or transplantation. Surgery. 2015;158:142–50.
Liu J, Liu J, Li J, Chen Y, Guan X, Wu X, et al. Tumor–stroma ratio is an independent predictor for survival in early cervical carcinoma. Gynecol Oncol. 2014;132:81–6.
Chen SX, Xu XE, Wang XQ, Cui SJ, Xu LL, Jiang YH, et al. Identification of colonic fibroblast secretomes reveals secretory factors regulating colon cancer cell proliferation. J Proteom. 2014;110:155–71.
Karagiannis GS, Berk A, Dimitromanolakis A, Diamandis EP. Enrichment map profiling of the cancer invasion front suggests regulation of colorectal cancer progression by the bone morphogenetic protein antagonist, gremlin-1. Mol Oncol. 2013;7:826–39.
Mesker WE, Liefers G-J, Junggeburt JMC, van Pelt GW, Alberici P, Kuppen PJK, et al. Presence of a high amount of stroma and downregulation of SMAD4 predict for worse survival for stage I-II colon cancer patients. Cell Oncol. 2009;31:169–78.
Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–84.
Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–43.
Alazzouzi H, Alhopuro P, Salovaara R, Sammalkorpi H, Jarvinen H, Mecklin JP et al. SMAD4 as a prognostic marker in colorectal cancer. Clin Cancer Res. 2005;11:2606–11. https://doi.org/10.1158/1078-0432.CCR-04-1458.
Kodach LL, Bleuming SA, Musler AR, Peppelenbosch MP, Hommes DW, van den Brink GR, et al. The bone morphogenetic protein pathway is active in human colon adenomas and inactivated in colorectal cancer. Cancer. 2008;112:300–6.
Alhopuro P, Alazzouzi H, Sammalkorpi H, Davalos V, Salovaara R, Hemminki A, et al. SMAD4 levels and response to 5-fluorouracil in colorectal cancer. Clin Cancer Res. 2005;11:6311–6.
Miyaki M, Iijima T, Konishi M, Sakai K, Ishii A, Yasuno M, et al. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene. 1999;18:3098–103.
Papageorgis P, Cheng K, Ozturk S, Gong Y, Lambert AW, Abdolmaleky HM, et al. Smad4 inactivation promotes malignancy and drug resistance of colon cancer. Cancer Res. 2011;71:998–1008.
Wakefield LM, Hill CS. Beyond TGFbeta: roles of other TGFbeta superfamily members in cancer. Nat Rev Cancer. 2013;13:328–41.
Hardwick JC, Van Den Brink GR, Bleuming SA, Ballester I, Van Den Brande JM, Keller JJ, et al. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004;126:111–21.
Kodach LL, Wiercinska E, de Miranda NF, Bleuming SA, Musler AR, Peppelenbosch MP, et al. The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Gastroenterology. 2008;134:1332–41.
Voorneveld PW, Kodach LL, Jacobs RJ, Liv N, Zonnevylle AC, Hoogenboom JP, et al. Loss of SMAD4 alters BMP signaling to promote colorectal cancer cell metastasis via activation of Rho and ROCK. Gastroenterology. 2014;147:196–208 e113.
Hardwick JC, Kodach LL, Offerhaus GJ, van den Brink GR. Bone morphogenetic protein signalling in colorectal cancer. Nat Rev Cancer. 2008;8:806–12.
Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17:79.
Irshad S, Bansal M, Guarnieri P, Davis H, Al Haj Zen A, Baran B, et al. Bone morphogenetic protein and Notch signalling crosstalk in poor-prognosis, mesenchymal-subtype colorectal cancer. J Pathol. 2017;242:178–92.
Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, et al. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008;27:6322.
Jiramongkolchai P, Owens P, Hong CC. Emerging roles of the bone morphogenetic protein pathway in cancer: potential therapeutic target for kinase inhibition. Biochem Soc Trans. 2016;44:1117–34.
Kleeff J, Maruyama H, Ishiwata T, Sawhney H, Friess H, Büchler MW, et al. Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology. 1999;116:1202–16.
Jin H, Pi J, Huang X, Huang F, Shao W, Li S, et al. BMP2 promotes migration and invasion of breast cancer cells via cytoskeletal reorganization and adhesion decrease: an AFM investigation. Appl Microbiol Biotechnol. 2012;93:1715–23.
Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL, Kim JS, et al. Metastatic function of BMP-2 in gastric cancer cells: the role of PI3K/AKT, MAPK, the NF-κB pathway, and MMP-9 expression. Exp Cell Res. 2011;317:1746–62.
Koch P-S, Olsavszky V, Ulbrich F, Sticht C, Demory A, Leibing T, et al. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood. 2017;129:415.
Lemke J, von Karstedt S, Zinngrebe J, Walczak H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014;21:1350.
Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782.
Koornstra JJ, Kleibeuker JH, van Geelen CMM, Rijcken FEM, Hollema H, de Vries EGE, et al. Expression of TRAIL (TNF-related apoptosis-inducing ligand) and its receptors in normal colonic mucosa, adenomas, and carcinomas. J Pathol. 2003;200:327–35.
Herzer K, Grosse-Wilde A, Krammer PH, Galle PR, Kanzler S. Transforming growth factor-β-mediated tumor necrosis factor-related apoptosis-inducing ligand expression and apoptosis in hepatoma cells requires functional cooperation between smad proteins and activator Protein-1. Mol Cancer Res. 2008;6:1169–77.
Cortez VS, Ulland TK, Cervantes-Barragan L, Bando JK, Robinette ML, Wang Q., et al. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-β signaling. Nature Immunology. 2017;18:995–1003.
Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Therapy. 2005;12:228–37.
Lane D, Cartier A, L’Espérance S, Côté‚ M, Rancourt C, Piché A. Differential induction of apoptosis by tumor necrosis factor-related apoptosis-inducing ligand in human ovarian carcinoma cells. Gynecologic Oncology. 2004;93:594–604.
Lee T-J, Lee JT, Park J-W, Kwon TK. Acquired TRAIL resistance in human breast cancer cells are caused by the sustained cFLIPL and XIAP proteinlevels and ERK activation. Biochemical and Biophysical Research Communications. 2006;351:1024–30.
Wenger T, Mattern J, Penzel R, Gassler N, Haas TL, Sprick MR., et al. Specific resistance upon lentiviral TRAIL transfer by intracellular retention of TRAIL receptors. Cell Death & Differentiation. 2006;13:1740–51.
Song JH, Tse MCL, Bellail A, Phuphanich S, Khuri F, Kneteman NM., et al. Lipid rafts and nonrafts mediate tumor necrosis factor-related apoptosis-inducing ligand-induced apoptotic and nonapoptotic signals in non-small cell lung carcinoma cells. Cancer Research. 2007;67:6946.
Hawinkels LJAC, ten Dijke P. Exploring anti-TGF-β therapies in cancer and fibrosis. Growth Factors. 2011;29:140–52.
Voorneveld PW, Stache V, Jacobs RJ, Smolders E, Sitters AI, Liesker A, et al. Reduced expression of bone morphogenetic protein receptor IA in pancreatic cancer is associated with a poor prognosis. Br J Cancer. 2013;109:1805–12.
Paauwe M, Schoonderwoerd MJA, Helderman RFCPA, Harryvan TJ, Groenewoud A, van Pelt GW et al. Endoglin expression on cancer-associated fibroblasts regulates invasion and stimulates colorectal cancer metastasis. Clin Cancer Res. 2018. https://doi.org/10.1158/1078-0432.CCR-18-0329.
Voorneveld PW, Kodach LL, Jacobs RJ, van Noesel CJM, Peppelenbosch MP, Korkmaz KS, et al. The BMP pathway either enhances or inhibits the Wnt pathway depending on the SMAD4 and p53 status in CRC. Br J Cancer. 2014;112:122.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ouahoud, S., Voorneveld, P.W., van der Burg, L.R.A. et al. Bidirectional tumor/stroma crosstalk promotes metastasis in mesenchymal colorectal cancer. Oncogene 39, 2453–2466 (2020). https://doi.org/10.1038/s41388-020-1157-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-1157-z
This article is cited by
-
Periostin–TGF-β feedforward loop contributes to tumour-stroma crosstalk in liver metastatic outgrowth of colorectal cancer
British Journal of Cancer (2024)
-
Tumor budding and fibrotic focus—proposed grading system for tumor budding in invasive carcinoma no special type of the breast
Virchows Archiv (2022)
-
Selective targeting BMP2 and 4 in SMAD4 negative esophageal adenocarcinoma inhibits tumor growth and aggressiveness in preclinical models
Cellular Oncology (2022)
-
Mechanisms of colorectal liver metastasis development
Cellular and Molecular Life Sciences (2022)
-
Tumour heterogeneity and evolutionary dynamics in colorectal cancer
Oncogenesis (2021)