Abstract
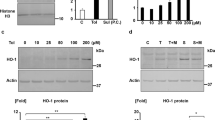
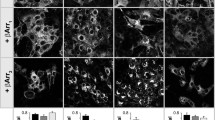
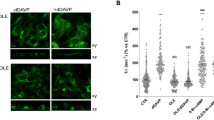
Arginine vasopressin (AVP) and its type-2 receptor (V2R) play an essential role in the regulation of salt and water homeostasis by the kidneys. V2R activation also stimulates proliferation of renal cell carcinoma (RCC) cell lines in vitro. The current studies investigated V2R expression and activity in human RCC tumors, and its role in RCC tumor growth. Examination of the cancer genome atlas (TCGA) database, and analysis of human RCC tumor tissue microarrays, cDNA arrays and tumor biopsy samples demonstrated V2R expression and activity in clear cell RCC (ccRCC). In vitro, V2R antagonists OPC31260 and Tolvaptan, or V2R gene silencing reduced wound closure and cell viability of 786-O and Caki-1 human ccRCC cell lines. Similarly in mouse xenograft models, Tolvaptan and OPC31260 decreased RCC tumor growth by reducing cell proliferation and angiogenesis, while increasing apoptosis. In contrast, the V2R agonist dDAVP significantly increased tumor growth. High intracellular cAMP levels and ERK1/2 activation were observed in human ccRCC tumors. In mouse tumors and Caki-1 cells, V2R agonists reduced cAMP and ERK1/2 activation, while dDAVP treatment had the reverse effect. V2R gene silencing in Caki-1 cells also reduced cAMP and ERK1/2 activation. These results provide novel evidence for a pathogenic role of V2R signaling in ccRCC, and suggest that inhibitors of the AVP-V2R pathway, including the FDA-approved drug Tolvaptan, could be utilized as novel ccRCC therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, et al. Renal cell carcinoma. Nat Rev Dis Prim. 2017;3:17009.
Boone M, Deen PM. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflug Arch. 2008;456:1005–24.
Keegan BP, Akerman BL, Pequeux C, North WG. Provasopressin expression by breast cancer cells: implications for growth and novel treatment strategies. Breast Cancer Res Treat. 2006;95:265–77.
North WG. Gene regulation of vasopressin and vasopressin receptors in cancer. Exp Physiol. 2000;85:27S–40S.
MacKinnon AC, Tufail-Hanif U, Lucas CD, Jodrell D, Haslett C, Sethi T. Expression of V1A and GRP receptors leads to cellular transformation and increased sensitivity to substance-P analogue-induced growth inhibition. Br J Cancer. 2005;92:522–31.
Pequeux C, Keegan BP, Hagelstein MT, Geenen V, Legros JJ, North WG. Oxytocin- and vasopressin-induced growth of human small-cell lung cancer is mediated by the mitogen-activated protein kinase pathway. Endocr Relat Cancer. 2004;11:871–85.
Garona J, Pifano M, Orlando UD, Pastrian MB, Iannucci NB, Ortega HH, et al. The novel desmopressin analogue [V4Q5]dDAVP inhibits angiogenesis, tumour growth and metastases in vasopressin type 2 receptor-expressing breast cancer models. Int J Oncol. 2015;46:2335–45.
Pifano M, Garona J, Capobianco CS, Gonzalez N, Alonso DF, Ripoll GV. Peptide agonists of vasopressin V2 receptor reduce expression of neuroendocrine markers and tumor growth in human lung and prostate tumor cells. Front Oncol. 2017;7:11.
Ripoll GV, Garona J, Pifano M, Farina HG, Gomez DE, Alonso DF. Reduction of tumor angiogenesis induced by desmopressin in a breast cancer model. Breast Cancer Res Treat. 2013;142:9–18.
Bolignano D, Medici MA, Coppolino G, Sciortino MT, Merlo FM, Campo S, et al. Aquaretic inhibits renal cancer proliferation: role of vasopressin receptor-2 (V2-R). Urologic Oncol. 2010;28:642–7.
Chen F, Zhang Y, Senbabaoglu Y, Ciriello G, Yang L, Reznik E, et al. Multilevel genomics-based taxonomy of renal cell carcinoma. Cell Rep. 2016;14:2476–89.
Davis CF, Ricketts CJ, Wang M, Yang L, Cherniack AD, Shen H, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell. 2014;26:319–30.
Prasad SR, Narra VR, Shah R, Humphrey PA, Jagirdar J, Catena JR, et al. Segmental disorders of the nephron: histopathological and imaging perspective. Br J Radio. 2007;80:593–602.
Wallace AC, Nairn RC. Renal tubular antigens in kidney tumors. Cancer. 1972;29:977–81.
Sarmiento JM, Ehrenfeld P, Anazco CC, Reyes CE, Troncoso S, Figueroa CD, et al. Differential distribution of the vasopressin V receptor along the rat nephron during renal ontogeny and maturation. Kidney Int. 2005;68:487–96.
Heasley LE. Autocrine and paracrine signaling through neuropeptide receptors in human cancer. Oncogene. 2001;20:1563–9.
Berl T, Quittnat-Pelletier F, Verbalis JG, Schrier RW, Bichet DG, Ouyang J, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrology. 2010;21:705–12.
Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, et al. A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrology. 2018;29:2458–70.
Ricketts CJ, De Cubas AA, Fan H, Smith CC, Lang M, Reznik E, et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 2018;23:3698.
Khan M, Huang T, Lin CY, Wu J, Fan BM, Bian ZX. Exploiting cancer’s phenotypic guise against itself: targeting ectopically expressed peptide G-protein coupled receptors for lung cancer therapy. Oncotarget. 2017;8:104615–37.
North WG, Fay MJ, Longo KA, Du J. Expression of all known vasopressin receptor subtypes by small cell tumors implies a multifaceted role for this neuropeptide. Cancer Res. 1998;58:1866–71.
Zhu X, Wess J. Truncated V2 vasopressin receptors as negative regulators of wild-type V2 receptor function. Biochemistry. 1998;37:15773–84.
Bunn PA, Jr., Chan D, Stewart J, Gera L, Tolley R, Jewett P, et al. Effects of neuropeptide analogues on calcium flux and proliferation in lung cancer cell lines. Cancer Res. 1994;54:3602–10.
Sethi T, Rozengurt E. Multiple neuropeptides stimulate clonal growth of small cell lung cancer: effects of bradykinin, vasopressin, cholecystokinin, galanin, and neurotensin. Cancer Res. 1991;51:3621–3.
Taylor AH, Ang VT, Jenkins JS, Silverlight JJ, Coombes RC, Luqmani YA. Interaction of vasopressin and oxytocin with human breast carcinoma cells. Cancer Res. 1990;50:7882–6.
Chooi KF, Carter DA, Biswas S, Lightman SL, Ho MY, Murphy D. Ectopic vasopressin expression in MMTV-Wnt-1 transgenic mice modifies mammary tumor differentiation and pathology. Cancer Res. 1994;54:6434–40.
North WG, Cole B, Akerman B, Pang RH. Growth impairment of small-cell cancer by targeting pro-vasopressin with MAG-1 antibody. Front Oncol. 2014;4:16.
North WG, Pang RH, Gao G, Memoli VA, Cole BF. Native MAG-1 antibody almost destroys human breast cancer xenografts. Breast Cancer Res Treat. 2011;127:631–7.
Bepler G, Carney DN, Gazdar AF, Minna JD. In vitro growth inhibition of human small cell lung cancer by physalaemin. Cancer Res. 1987;47:2371–5.
Bunn PA, Jr., Chan D, Dienhart DG, Tolley R, Tagawa M, Jewett PB. Neuropeptide signal transduction in lung cancer: clinical implications of bradykinin sensitivity and overall heterogeneity. Cancer Res. 1992;52:24–31.
Schwindt TT, Forti FL, Juliano MA, Juliano L, Armelin HA. Arginine vasopressin inhibition of cyclin D1 gene expression blocks the cell cycle and cell proliferation in the mouse Y1 adrenocortical tumor cell line. Biochemistry. 2003;42:2116–21.
Thibonnier M, Conarty DM, Plesnicher CL. Mediators of the mitogenic action of human V(1) vascular vasopressin receptors. Am J Physiol Heart Circ Physiol. 2000;279:H2529–39.
Cho-Chung YS. Suppression of malignancy targeting cyclic AMP signal transducing proteins. Biochem Soc Trans. 1992;20:425–30.
Dicitore A, Grassi ES, Caraglia M, Borghi MO, Gaudenzi G, Hofland LJ, et al. The cAMP analogs have potent anti-proliferative effects on medullary thyroid cancer cell lines. Endocrine. 2016;51:101–12.
Kim SN, Ahn YH, Kim SG, Park SD, Cho-Chung YS, Hong SH. 8-Cl-cAMP induces cell cycle-specific apoptosis in human cancer cells. Int J Cancer. 2001;93:33–41.
Rocha AS, Paternot S, Coulonval K, Dumont JE, Soares P, Roger PP. Cyclic AMP inhibits the proliferation of thyroid carcinoma cell lines through regulation of CDK4 phosphorylation. Mol Biol Cell. 2008;19:4814–25.
Shaw TJ, Keszthelyi EJ, Tonary AM, Cada M, Vanderhyden BC. Cyclic AMP in ovarian cancer cells both inhibits proliferation and increases c-KIT expression. Exp Cell Res. 2002;273:95–106.
Dremier S, Coulonval K, Perpete S, Vandeput F, Fortemaison N, Van Keymeulen A, et al. The role of cyclic AMP and its effect on protein kinase A in the mitogenic action of thyrotropin on the thyroid cell. Ann N Y Acad Sci. 2002;968:106–21.
Rodrigues AL, Brescia M, Koschinski A, Moreira TH, Cameron RT, Baillie G, et al. Increase in Ca(2+) current by sustained cAMP levels enhances proliferation rate in GH3 cells. Life Sci. 2018;192:144–50.
Starzec AB, Spanakis E, Nehme A, Salle V, Veber N, Mainguene C, et al. Proliferative responses of epithelial cells to 8-bromo-cyclic AMP and to a phorbol ester change during breast pathogenesis. J Cell Physiol. 1994;161:31–8.
Takahashi H, Honma M, Miyauchi Y, Nakamura S, Ishida-Yamamoto A, Iizuka H. Cyclic AMP differentially regulates cell proliferation of normal human keratinocytes through ERK activation depending on the expression pattern of B-Raf. Arch Dermatol Res. 2004;296:74–82.
Tierney T, Robinson IC. Increased lactotrophs despite decreased somatotrophs in the dwarf (dw/dw) rat: a defect in the regulation of lactotroph/somatotroph cell fate? J Endocrinol. 2002;175:435–46.
Vitali E, Peverelli E, Giardino E, Locatelli M, Lasio GB, Beck-Peccoz P, et al. Cyclic adenosine 3’-5’-monophosphate (cAMP) exerts proliferative and anti-proliferative effects in pituitary cells of different types by activating both cAMP-dependent protein kinase A (PKA) and exchange proteins directly activated by cAMP (Epac). Mol Cell Endocrinol. 2014;383:193–202.
Zivadinovic D, Gametchu B, Watson CS. Membrane estrogen receptor-alpha levels in MCF-7 breast cancer cells predict cAMP and proliferation responses. Breast Cancer Res. 2005;7:R101–12.
Alonso DF, Skilton G, Farias EF, Bal de Kier Joffe E, Gomez DE. Antimetastatic effect of desmopressin in a mouse mammary tumor model. Breast Cancer Res Treat. 1999;57:271–5.
Garona J, Sobol NT, Pifano M, Segatori VI, Gomez DE, Ripoll GV. et al. Preclinical efficacy of [V4Q5]dDAVP, a second generation vasopressin analog, on metastatic spread and tumor-associated angiogenesis in colorectal cancer. Cancer Res Treat. 2019;51:438–50.
Giron S, Tejera AM, Ripoll GV, Gomez DE, Alonso DF. Desmopressin inhibits lung and lymph node metastasis in a mouse mammary carcinoma model of surgical manipulation. J Surg Oncol. 2002;81:38–44.
Iannucci NB, Ripoll GV, Garona J, Cascone O, Ciccia GN, Gomez DE, et al. Antiproliferative effect of 1-deamino-8-D-arginine vasopressin analogs on human breast cancer cells. Future Med Chem. 2011;3:1987–93.
Zaoral M. Vasopressin analogs with high and specific antidiuretic activity. Int J Pept Protein Res. 1985;25:561–74.
Ryan MB, Der CJ, Wang-Gillam A, Cox AD. Targeting RAS-mutant cancers: is ERK the key? Trends Cancer. 2015;1:183–98.
Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:103–19.
Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59:561–74.
Huang D, Ding Y, Luo WM, Bender S, Qian CN, Kort E, et al. Inhibition of MAPK kinase signaling pathways suppressed renal cell carcinoma growth and angiogenesis in vivo. Cancer Res. 2008;68:81–8.
Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12:7271–8.
Cai Q, McReynolds MR, Keck M, Greer KA, Hoying JB, Brooks HL. Vasopressin receptor subtype 2 activation increases cell proliferation in the renal medulla of AQP1 null mice. Am J Physiol Ren Physiol. 2007;293:F1858–64.
Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem. 2004;279:40419–30.
Tao S, Kakade VR, Woodgett JR, Pandey P, Suderman ED, Rajagopal M, et al. Glycogen synthase kinase-3beta promotes cyst expansion in polycystic kidney disease. Kidney Int. 2015;87:1164–75.
Seeger-Nukpezah T, Geynisman DM, Nikonova AS, Benzing T, Golemis EA. The hallmarks of cancer: relevance to the pathogenesis of polycystic kidney disease. Nat Rev Nephrol. 2015;11:515–34.
Yamamura Y, Ogawa H, Yamashita H, Chihara T, Miyamoto H, Nakamura S, et al. Characterization of a novel aquaretic agent, OPC-31260, as an orally effective, nonpeptide vasopressin V2 receptor antagonist. Br J Pharmacol. 1992;105:787–91.
Kondo K, Ogawa H, Yamashita H, Miyamoto H, Tanaka M, Nakaya K, et al. 7-Chloro-5-hydroxy-1-[2-methyl-4-(2-methylbenzoyl-amino)benzoyl]-2,3,4,5-tetrahydro-1H-1-benzazepine (OPC-41061): a potent, orally active nonpeptide arginine vasopressin V2 receptor antagonist. Bioorg Med Chem. 1999;7:1743–54.
Shoaf SE, Kim SR, Bricmont P, Mallikaarjun S. Pharmacokinetics and pharmacodynamics of single-dose oral tolvaptan in fasted and non-fasted states in healthy Caucasian and Japanese male subjects. Eur J Clin Pharmacol. 2012;68:1595–603.
Aihara M, Fujiki H, Mizuguchi H, Hattori K, Ohmoto K, Ishikawa M, et al. Tolvaptan delays the onset of end-stage renal disease in a polycystic kidney disease model by suppressing increases in kidney volume and renal injury. J Pharmacol Exp Ther. 2014;349:258–67.
Watkins PB, Lewis JH, Kaplowitz N, Alpers DH, Blais JD, Smotzer DM, et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf. 2015;38:1103–13.
Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP, Vikas P. The repurposing drugs in oncology (ReDO) project. Ecancermedicalscience. 2014;8:442.
Seo EJ, Sugimoto Y, Greten HJ, Efferth T. Repurposing of bromocriptine for cancer therapy. Front Pharmacol. 2018;9:1030.
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. New Engl J Med. 2017;377:1930–42.
Burst V, Grundmann F, Kubacki T, Greenberg A, Rudolf D, Salahudeen A, et al. Euvolemic hyponatremia in cancer patients. Report of the Hyponatremia Registry: an observational multicenter international study. Support Care Cancer. 2017;25:2275–83.
Onitilo AA, Kio E, Doi SA. Tumor-related hyponatremia. Clin Med Res. 2007;5:228–37.
Bellmunt J, Leow JJ. Hyponatremia associated with worse outcomes in metastatic renal cell cancer: a potential target for intervention? Eur Urol. 2014;65:731–2.
Jeppesen AN, Jensen HK, Donskov F, Marcussen N, von der Maase H. Hyponatremia as a prognostic and predictive factor in metastatic renal cell carcinoma. Br J Cancer. 2010;102:867–72.
Gralla RJ, Ahmad F, Blais JD, Chiodo J 3rd, Zhou W, Glaser LA, et al. Tolvaptan use in cancer patients with hyponatremia due to the syndrome of inappropriate antidiuretic hormone: a post hoc analysis of the SALT-1 and SALT-2 trials. Cancer Med. 2017;6:723–9.
R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. R Foundation for Statistical Computing, Vienna; 2014.
Kakade VR, Tao S, Rajagopal M, Zhou X, Li X, Yu AS, et al. A cAMP and CREB-mediated feed-forward mechanism regulates GSK3beta in polycystic kidney disease. J Mol Cell Biol. 2016;8:464–76.
Rao R, Patel S, Hao C, Woodgett J, Harris R. GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity. J Am Soc Nephrology. 2010;21:428–37.
Shi PP, Cao XR, Qu J, Volk KA, Kirby P, Williamson RA, et al. Nephrogenic diabetes insipidus in mice caused by deleting COOH-terminal tail of aquaporin-2. Am J Physiol Ren Physiol. 2007;292:F1334–44.
Singh SP, Tao S, Fields TA, Webb S, Harris RC, Rao R. Glycogen synthase kinase-3 inhibition attenuates fibroblast activation and development of fibrosis following renal ischemia-reperfusion in mice. Dis Models Mech. 2015;8:931–40.
Gunaratne R, Braucht DW, Rinschen MM, Chou CL, Hoffert JD, Pisitkun T, et al. Quantitative phosphoproteomic analysis reveals cAMP/vasopressin-dependent signaling pathways in native renal thick ascending limb cells. Proc Natl Acad Sci USA. 2010;107:15653–8.
Acknowledgements
We thank the University of Kansas Cancer Center Biospecimen Core (P30 CA168524) and the PKD Biomarkers and Biomaterials Core (P30-DK106912) for human specimens, and the Flow Cytometry Core (P30 GM103326 NIH/NIGMS COBRE grant) of University of Kansas Medical Center.
Funding
This study was supported by NIH R01-DK083525, a private donation from the Watts family and a Pilot and Feasibility Grant from the KU Cancer Center and PKD Research and Translation Core Center P30-DK106912 to RR. ND is supported by Postdoctoral Fellowship Grants from the KU Biomedical Research and Training Program and American Heart Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sinha, S., Dwivedi, N., Tao, S. et al. Targeting the vasopressin type-2 receptor for renal cell carcinoma therapy. Oncogene 39, 1231–1245 (2020). https://doi.org/10.1038/s41388-019-1059-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-1059-0
This article is cited by
-
AVPR2 is a potential prognostic biomarker and correlated with immune infiltration in head and neck squamous cell carcinoma
BMC Medical Genomics (2023)
-
Vasopressin induces apoptosis but does not enhance the antiproliferative effect of dynamin 2 or PI3K/Akt inhibition in luminal A breast cancer cells
Medical Oncology (2022)
-
The V2 receptor antagonist tolvaptan counteracts proliferation and invasivity in human cancer cells
Journal of Endocrinological Investigation (2022)
-
Identification of an independent immune-genes prognostic index for renal cell carcinoma
BMC Cancer (2021)
-
The tyrosine-kinase inhibitor Nintedanib ameliorates autosomal-dominant polycystic kidney disease
Cell Death & Disease (2021)