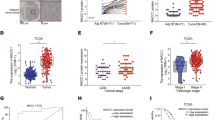
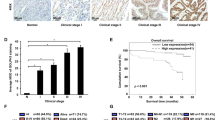
Abstract
Colorectal cancer (CRC) is a common gastrointestinal cancer with high mortality rate mostly due to metastasis. Ca2+-dependent activator protein for secretion 1 (CAPS1) was originally identified as a soluble factor that reconstitutes Ca2+-dependent secretion. In this study, we discovered a novel role of CAPS1 in CRC metastasis. CAPS1 is frequently up-regulated in CRC tissues. Increased CAPS1 expression is associated with frequent metastasis and poor prognosis of CRC patients. Overexpression of CAPS1 promotes CRC cell migration and invasion in vitro, as well as liver metastasis in vivo, without affecting cell proliferation. CAPS1 induces epithelial–mesenchymal transition (EMT), including decreased E-cadherin and ZO-1, epithelial marker expression, and increased N-cadherin and Snail, mesenchymal marker expression. Snail knockdown reversed CAPS1-induced EMT, cell migration and invasion. This result indicates that Snail is required for CAPS1-mediated EMT process and metastasis in CRC. Furthermore, CAPS1 can bind with Septin2 and p85 (subunit of PI3K). LY294002 and wortmanin, PI3K/Akt inhibitors, can abolish CAPS1-induced increase of Akt/GSK3β activity, as well as increase of Snail protein level. Taken together, CAPS1 promotes colorectal cancer metastasis through PI3K/Akt/GSK3β/Snail signal pathway-mediated EMT process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Mundade R, Imperiale TF, Prabhu L, Loehrer PJ, Lu T. Genetic pathways, prevention, and treatment of sporadic colorectal cancer. Oncoscience. 2014;1:400–6.
Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378–91.
Cao H, Xu E, Liu H, Wan L, Lai M. Epithelial-mesenchymal transition in colorectal cancer metastasis: a system review. Pathol Res Pract. 2015;211:557–69.
Vu T, Datta PK. Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers (Basel). 2017;9:pii: E171
Wang J, Zhu X, Hu J, He G, Li X, Wu P, et al. The positive feedback between Snail and DAB2IP regulates EMT, invasion and metastasis in colorectal cancer. Oncotarget. 2015;6:27427–39.
Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–40.
Lu LL, Chen XH, Zhang G, Liu ZC, Wu N, Wang H, et al. CCL21 facilitates chemoresistance and cancer stem cell-like properties of colorectal cancer cells through AKT/GSK-3beta/Snail signals. Oxid Med Cell Longev. 2016;2016:5874127.
Mali AV, Joshi AA, Hegde MV, Kadam SS. Enterolactone modulates the ERK/NF-kappaB/Snail signaling pathway in triple-negative breast cancer cell line MDA-MB-231 to revert the TGF-beta-induced epithelial-mesenchymal transition. Cancer Biol Med. 2018;15:137–56.
Luo W, Liu X, Lu JJ, Wang Y, Chen X. Toosendanin, a natural product, inhibited TGF-beta1-induced epithelial-mesenchymal transition through ERK/Snail pathway. Phytother Res. 2018;32:2009–20.
Rokavec M, Oner MG, Li H, Jackstadt R, Jiang L, Lodygin D, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124:1853–67.
Tang BL. A unique SNARE machinery for exocytosis of cytotoxic granules and platelets granules. Mol Membr Biol. 2015;32:120–6.
Tanaka T, Goto K, Iino M. Diverse functions and signal transduction of the exocyst complex in tumor cells. J Cell Physiol. 2017;232:939–57.
Wu LG, Hamid E, Shin W, Chiang HC. Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol. 2014;76:301–31.
Chen L, Guo P, He Y, Chen Z, Chen L, Luo Y, et al. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018;9:513.
Mathias RA, Wang B, Ji H, Kapp EA, Moritz RL, Zhu HJ, et al. Secretome-based proteomic profiling of Ras-transformed MDCK cells reveals extracellular modulators of epithelial-mesenchymal transition. J Proteome Res. 2009;8:2827–37.
Walent JH, Porter BW, Martin TF. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992;70:765–75.
Rettig J, Neher E. Emerging roles of presynaptic proteins in Ca++-triggered exocytosis. Science. 2002;298:781–5.
Hay JC, Martin TF. Resolution of regulated secretion into sequential MgATP-dependent and calcium-dependent stages mediated by distinct cytosolic proteins. J Cell Biol. 1992;119:139–51.
Ann K, Kowalchyk JA, Loyet KM, Martin TF. Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J Biol Chem. 1997;272:19637–40.
Koch H, Hofmann K, Brose N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem J. 2000;349:247–53.
James DJ, Kowalchyk J, Daily N, Petrie M, Martin TF. CAPS drives trans-SNARE complex formation and membrane fusion through syntaxin interactions. Proc Natl Acad Sci USA. 2009;106:17308–13.
Sadakata T, Washida M, Morita N, Furuichi T. Tissue distribution of Ca2+-dependent activator protein for secretion family members CAPS1 and CAPS2 in mice. J Histochem Cytochem. 2007;55:301–11.
Hosono M, Shinoda Y, Hirano T, Ishizaki Y, Furuichi T, Sadakata T. Interaction of Ca(2+)-dependent activator protein for secretion 1 (CAPS1) with septin family proteins in mouse brain. Neurosci Lett. 2016;617:232–5.
Garcia Z, Silio V, Marques M, Cortes I, Kumar A, Hernandez C, et al. A PI3K activity-independent function of p85 regulatory subunit in control of mammalian cytokinesis. EMBO J. 2006;25:4740–51.
Chen T, Li J, Xu M, Zhao Q, Hou Y, Yao L, et al. PKCepsilon phosphorylates MIIP and promotes colorectal cancer metastasis through inhibition of RelA deacetylation. Nat Commun. 2017;8:939.
Im HJ, Kim HG, Lee JS, Kim HS, Cho JH, Jo IJ, et al. A preclinical model of chronic alcohol consumption reveals increased metastatic seeding of colon cancer cells in the liver. Cancer Res. 2016;76:1698–704.
VanSaun MN, Lee IK, Washington MK, Matrisian L, Gorden DL. High fat diet induced hepatic steatosis establishes a permissive microenvironment for colorectal metastases and promotes primary dysplasia in a murine model. Am J Pathol. 2009;175:355–64.
Zhang Y, Davis C, Shah S, Hughes D, Ryan JC, Altomare D, et al. IL-33 promotes growth and liver metastasis of colorectal cancer in mice by remodeling the tumor microenvironment and inducing angiogenesis. Mol Carcinog. 2017;56:272–87.
Miller S, Rogers HA, Lyon P, Rand V, Adamowicz-Brice M, Clifford SC, et al. Genome-wide molecular characterization of central nervous system primitive neuroectodermal tumor and pineoblastoma. Neuro Oncol. 2011;13:866–79.
Liu T, Xue R, Huang X, Zhang D, Dong L, Wu H, et al. Proteomic profiling of hepatitis B virus-related hepatocellular carcinoma with magnetic bead-based matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Acta Biochim Biophys Sin (Shanghai). 2011;43:542–50.
Xue R, Tang W, Dong P, Weng S, Ma L, Chen S. et al. CAPS1 negatively regulates hepatocellular carcinoma development through alteration of exocytosis-associated tumor microenvironment. Int J Mol Sci. 2016;17:pii: E1626
Vleminckx K, Vakaet L Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–19.
Chen XF, Zhang HJ, Wang HB, Zhu J, Zhou WY, Zhang H, et al. Transforming growth factor-beta1 induces epithelial-to-mesenchymal transition in human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling pathways. Mol Biol Rep. 2012;39:3549–56.
Nagano K, Takeuchi H, Gao J, Mori Y, Otani T, Wang D, et al. Tomosyn is a novel Akt substrate mediating insulin-dependent GLUT4 exocytosis. Int J Biochem Cell Biol. 2015;62:62–71.
Pemberton JG, Orr ME, Stafford JL, Chang JP. PI3K signalling in GnRH actions on dispersed goldfish pituitary cells: relationship with PKC-mediated LH and GH release and regulation of long-term effects on secretion and total cellular hormone availability. Gen Comp Endocrinol. 2014;205:268–78.
Leipe DD, Wolf YI, Koonin EV, Aravind L. Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol. 2002;317:41–72.
Huang YW, Surka MC, Reynaud D, Pace-Asciak C, Trimble WS. GTP binding and hydrolysis kinetics of human septin 2. FEBS J. 2006;273:3248–60.
Abbey M, Hakim C, Anand R, Lafera J, Schambach A, Kispert A, et al. GTPase domain driven dimerization of SEPT7 is dispensable for the critical role of septins in fibroblast cytokinesis. Sci Rep. 2016;6:20007.
Angelis D, Spiliotis ET. Septin mutations in human cancers. Front Cell Dev Biol. 2016;4:122.
Xu D, Liu A, Wang X, Chen Y, Shen Y, Tan Z, et al. Repression of Septin9 and Septin2 suppresses tumor growth of human glioblastoma cells. Cell Death Dis. 2018;9:514.
Cao LQ, Shao ZL, Liang HH, Zhang DW, Yang XW, Jiang XF, et al. Activation of peroxisome proliferator-activated receptor-gamma (PPARgamma) inhibits hepatoma cell growth via downregulation of SEPT2 expression. Cancer Lett. 2015;359:127–35.
Huang X, Xiang L, Li Y, Zhao Y, Zhu H, Xiao Y, et al. Snail/FOXK1/Cyr61 signaling axis regulates the epithelial-mesenchymal transition and metastasis in colorectal cancer. Cell Physiol Biochem. 2018;47:590–603.
Wang Y, Wu Z, Hu L. The regulatory effects of metformin on the [SNAIL/miR-34]:[ZEB/miR-200] system in the epithelial-mesenchymal transition(EMT) for colorectal cancer(CRC). Eur J Pharmacol. 2018;834:45–53.
Hahn S, Jackstadt R, Siemens H, Hunten S, Hermeking H. SNAIL and miR-34a feed-forward regulation of ZNF281/ZBP99 promotes epithelial-mesenchymal transition. EMBO J. 2013;32:3079–95.
Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28.
Sanchez-Tillo E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, et al. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69:3429–56.
Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–3.
Shiozaki H, Oka H, Inoue M, Tamura S, Monden M. E-cadherin mediated adhesion system in cancer cells. Cancer. 1996;77:1605–13.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89.
Thuault S, Tan EJ, Peinado H, Cano A, Heldin CH, Moustakas A. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J Biol Chem. 2008;283:33437–46.
Smit MA, Geiger TR, Song JY, Gitelman I, Peeper DS. A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol. 2009;29:3722–37.
Guaita S, Puig I, Franci C, Garrido M, Dominguez D, Batlle E, et al. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression and ZEB1 expression. J Biol Chem. 2002;277:39209–16.
Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15:569–79.
Agarwal E, Chaudhuri A, Leiphrakpam PD, Haferbier KL, Brattain MG, Chowdhury S. Akt inhibitor MK-2206 promotes anti-tumor activity and cell death by modulation of AIF and Ezrin in colorectal cancer. BMC Cancer. 2014;14:145.
Do K, Speranza G, Bishop R, Khin S, Rubinstein L, Kinders RJ, et al. Biomarker-driven phase 2 study of MK-2206 and selumetinib (AZD6244, ARRY-142886) in patients with colorectal cancer. Invest New Drugs. 2015;33:720–8.
Acknowledgements
We thank Dr. Hai-Ying Zeng from the Department of Pathology, Zhongshan Hospital of Fudan University, for technical assistance.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (81672334, 81772615, 81572686, 81772968, 81573423, 81770137, 81672720) and Shanghai Science and Technology Commission (15410710100, 16ZR1406100).
Author contributions
SC, LD, XS, and GZ designed and conceived this project. GZ and SC developed methodology. GZ, YX, SW, and YC performed experiments and generated data. YX, SC, and SZ analyzed and interpreted data. YX, SC, and GZ wrote the manuscript. All authors contributed to and approved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, GX., Xu, YY., Weng, SQ. et al. CAPS1 promotes colorectal cancer metastasis via Snail mediated epithelial mesenchymal transformation. Oncogene 38, 4574–4589 (2019). https://doi.org/10.1038/s41388-019-0740-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0740-7
This article is cited by
-
PI3K/AKT signaling pathway as a critical regulator of epithelial-mesenchymal transition in colorectal tumor cells
Cell Communication and Signaling (2023)
-
LINC02870 facilitates SNAIL translation to promote hepatocellular carcinoma progression
Molecular and Cellular Biochemistry (2023)
-
CAPS1 Suppresses Tumorigenesis in Cholangiocarcinoma
Digestive Diseases and Sciences (2020)