Abstract
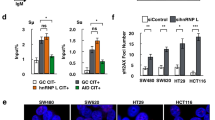
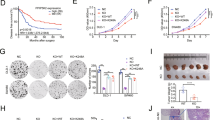
A multicenter clinical study demonstrated the presence of a loss-of-function HSP110 mutation in about 15% of colorectal cancers, which resulted from an alternative splicing and was produced at the detriment of wild-type HSP110. Patients expressing low levels of wild-type HSP110 had excellent outcomes (i.e. response to an oxaliplatin-based chemotherapy). Here, we show in vitro, in vivo, and in patients’ biopsies that HSP110 co-localizes with DNA damage (γ-H2AX). In colorectal cancer cells, HSP110 translocates into the nucleus upon treatment with genotoxic chemotherapy such as oxaliplatin. Furthermore, we show that HSP110 interacts with the Ku70/Ku80 heterodimer, an essential element of the non-homologous end joining (NHEJ) repair machinery. We also demonstrate by evaluating the resolved 53BP1 foci that depletion in HSP110 impairs repair steps of the NHEJ pathway, which is associated with an increase in DNA double-strand breaks and in the cells’ sensitivity to oxaliplatin. HSP110-depleted cells sensitization to oxaliplatin-induced DNA damage is abolished upon re-expression of HSP110. Confirming a role for HSP110 in DNA non-homologous repair, SCR7 and NU7026, two inhibitors of the NHEJ pathway, circumvents HSP110-induced resistance to chemotherapy. In conclusion, HSP110 through its interaction with the Ku70/80 heterodimer may participate in DNA repair, thereby inducing a protection against genotoxic therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–72.
Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–601.
Guttmann DM, Koumenis C. The heat shock proteins as targets for radiosensitization and chemosensitization in cancer. Cancer Biol Ther. 2011;12:1023–31.
Collura A, Lagrange A, Svrcek M, Marisa L, Buhard O, Guilloux A, et al. Patients with colorectal tumors with microsatellite instability and large deletions in HSP110 T17 have improved response to 5-fluorouracil-based chemotherapy. Gastroenterology. 2014;146:401–11 e1.
Dorard C, de Thonel A, Collura A, Marisa L, Svrcek M, Lagrange A, et al. Expression of a mutant HSP110 sensitizes colorectal cancer cells to chemotherapy and improves disease prognosis. Nat Med. 2011;17:1283–9.
Kimura A, Ogata K, Altan B, Yokobori T, Ide M, Mochiki E, et al. Nuclear heat shock protein 110 expression is associated with poor prognosis and chemotherapy resistance in gastric cancer. Oncotarget. 2016;7:18415–23.
Mattoo RUH, Sharma SK, Priya S, Finka A, Goloubinoff P. Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J Biol Chem. 2013;288:21399–411.
Rampelt H, Kirstein-Miles J, Nillegoda NB, Chi K, Scholz SR, Morimoto RI, et al. Metazoan Hsp70 machines use Hsp110 to power protein disaggregation. EMBO J. 2012;31:4221–35.
Berthenet K, Bokhari A, Lagrange A, Marcion G, Boudesco C, Causse S. et al. HSP110 promotes colorectal cancer growth through STAT3 activation. Oncogene. 2017;36:2328–36.
Yu N, Kakunda M, Pham V, Lill JR, Du P, Wongchenko M, et al. HSP105 recruits protein phosphatase 2A to dephosphorylate β-catenin. Mol Cell Biol. 2015;35:1390–400.
Mao Z, Bozzella M, Seluanov A, Gorbunova V. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle. 2008;7:2902–6.
Panier S, Boulton SJ. Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol. 2014;15:7–18.
Willmore E, de Caux S, Sunter NJ, Tilby MJ, Jackson GH, Austin CA, et al. A novel DNA-dependent protein kinase inhibitor, NU7026, potentiates the cytotoxicity of topoisomerase II poisons used in the treatment of leukemia. Blood. 2004;103:4659–65.
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–72.
Kai M, Nakatsura T, Egami H, Senju S, Nishimura Y, Ogawa M. Heat shock protein 105 is overexpressed in a variety of human tumors. Oncol Rep. 2003;10:1777–82.
Thomas X, Campos L, Mounier C, Cornillon J, Flandrin P, Le Q-H, et al. Expression of heat-shock proteins is associated with major adverse prognostic factors in acute myeloid leukemia. Leuk Res. 2005;29:1049–58.
Yang Z, Zhuang L, Szatmary P, Wen L, Sun H, Lu Y, et al. Upregulation of heat shock proteins (HSPA12A, HSP90B1, HSPA4, HSPA5 and HSPA6) in tumour tissues is associated with poor outcomes from HBV-related early-stage hepatocellular carcinoma. Int J Med Sci. 2015;12:256–63.
Saito Y, Yamagishi N, Hatayama T. Different localization of Hsp105 family proteins in mammalian cells. Exp Cell Res. 2007;313:3707–17.
Gravina GL, Senapedis W, McCauley D, Baloglu E, Shacham S, Festuccia C. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol. 2014;7:85.
Dufour A, Thibeaux R, Labruyère E, Guillén N, Olivo-Marin J-C. 3-D active meshes: fast discrete deformable models for cell tracking in 3-D time-lapse microscopy. IEEE Trans Image Process. 2011;20:1925–37.
Olivo-Marin J-C. Extraction of spots in biological images using multiscale products. Pattern Recognit. 2002;35:1989–96.
Acknowledgements
We are grateful professor Gorbunova for allowing us using her NHEJ DNA repair system and to Alex Duval and Ada Collura for helpful discussions. We also thank the Platforms of cytometry Cellimap. This work was supported by grants from the Institut National du Cancer, Ligue Nationale Contre le Cancer, and the Conseil Régional de Bourgogne. The work was also supported by a French Government grant managed by the French National Research Agency under the program “Investissements d’Avenir” with reference ANR-11-LABX-0021 (LabEX LipSTIC). We thank the European Union programme FEDER for their financial support. SC had a fellowship from La Ligue Nationale contre le Cancer, GM from the Association pour la Recherche sur le Cancer, GG from the «Fondation de France», and GC from the «Fondation pour la Recherche Médicale (ECO20160736090)».
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Causse, S.Z., Marcion, G., Chanteloup, G. et al. HSP110 translocates to the nucleus upon genotoxic chemotherapy and promotes DNA repair in colorectal cancer cells. Oncogene 38, 2767–2777 (2019). https://doi.org/10.1038/s41388-018-0616-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0616-2
This article is cited by
-
Knockdown of HSP110 attenuates hypoxia-induced pulmonary hypertension in mice through suppression of YAP/TAZ-TEAD4 pathway
Respiratory Research (2022)
-
Cancer-associated fibroblast-induced lncRNA UPK1A-AS1 confers platinum resistance in pancreatic cancer via efficient double-strand break repair
Oncogene (2022)
-
Diversity in heat shock protein families: functional implications in virus infection with a comprehensive insight of their role in the HIV-1 life cycle
Cell Stress and Chaperones (2021)
-
Heat-shock proteins: chaperoning DNA repair
Oncogene (2020)