Abstract
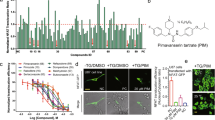
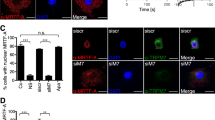
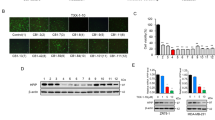
Glioblastomas (GBM) are the most aggressive brain cancers without effective therapeutics. The Hippo pathway transcriptional coactivators YAP/TAZ were implicated as drivers in GBM progression and could be therapeutic targets. Here we found in an unbiased screen of 1650 compounds that amlodipine is able to inhibit survival of GBM cells by suppressing YAP/TAZ activities. Instead of its known function as an L-type calcium channel blocker, we found that amlodipine is able to activate Ca2+ entry by enhancing store-operated Ca2+ entry (SOCE). Amlodipine as well as approaches that cause store depletion and activate SOCE trigger phosphorylation and activation of Lats1/2, which in turn phosphorylate YAP/TAZ and prevent their accumulation in the cell nucleus. Furthermore, we identified that protein kinase C (PKC) beta II is a major mediator of Ca2+-induced Lats1/2 activation. Ca2+ induces accumulation of PKC beta II in an actin cytoskeletal compartment. Such translocation depends on inverted formin-2 (INF2). Depletion of INF2 disrupts both PKC beta II translocation and Lats1/2 activation. Functionally, we found that elevation of cytosolic Ca2+ or PKC beta II expression inhibits YAP/TAZ-mediated gene transcription. In vivo PKC beta II expression inhibits GBM tumor growth and prolongs mouse survival through inhibition of YAP/TAZ in an orthotopic mouse xenograft model. Our studies indicate that Ca2+ is a crucial intracellular cue that regulates the Hippo pathway and that triggering SOCE could be a strategy to target YAP/TAZ in GBM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108.
Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110.
Bhat KP, Salazar KL, Balasubramaniyan V, Wani K, Heathcock L, Hollingsworth F, et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev. 2011;25:2594–609.
Orr BA, Bai H, Odia Y, Jain D, Anders RA, Eberhart CG. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J Neuropathol Exp Neurol. 2011;70:568–77.
Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–28.
Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57.
Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803.
Tian T, Li A, Lu H, Luo R, Zhang M, Li Z. TAZ promotes temozolomide resistance by upregulating MCL-1 in human glioma cells. Biochem Biophys Res Commun. 2015;463:638–43.
Fernandez LA, Squatrito M, Northcott P, Awan A, Holland EC, Taylor MD, et al. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31:1923–37.
Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–97.
Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–84.
Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79.
Monteith GR, Prevarskaya N, Roberts-Thomson SJ. The calcium-cancer signalling nexus. Nat Rev Cancer. 2017;17:367–80.
Roderick HL, Cook SJ. Ca2+signalling checkpoints in cancer: remodelling Ca2+for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8:361–75.
Potier M, Trebak M. New developments in the signaling mechanisms of the store-operated calcium entry pathway. Pflugers Arch. 2008;457:405–15.
Trebak M, Putney JW. ORAI calcium channels. Physiology. 2017;32:332–42.
Jardin I, Rosado JA. STIM and calcium channel complexes in cancer. Biochim Biophys Acta. 2016;1863:1418–26.
Li W, Dong S, Wei W, Wang G, Zhang A, Pu P, et al. The role of transcriptional coactivator TAZ in gliomas. Oncotarget. 2016;7:82686–99.
Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the Hippo pathway. Mol Cell Biol. 2008;28:2426–36.
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61.
DeHaven WI, Smyth JT, Boyles RR, Bird GS, Putney JW. Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J Biol Chem. 2008;283:19265–73.
Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+release-activated Ca2+channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol. 2001;536:3–19.
Trebak M, Bird GSJ, McKay RR, Putney JW. Comparison of human TRPC3 channels in receptor-activated and store-operated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition. J Biol Chem. 2002;277:21617–23.
Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–99.
Smyth JT, DeHaven WI, Bird GS, Putney JW. Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci. 2008;121:762–72.
Morgan AJ, Jacob R. Ionomycin enhances Ca2+influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem J. 1994;300(Pt 3):665–72.
Takemura H, Hughes AR, Thastrup O, Putney JW. Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool, and not an inositol phosphate, regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989;264:12266–71.
Motiani RK, Hyzinski-Garcia MC, Zhang X, Henkel MM, Abdullaev IF, Kuo YH, et al. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch. 2013;465:1249–60.
Asai M, Takeuchi K, Uchida S, Urushida T, Katoh H, Satoh H, et al. Misinterpretation of the effect of amlodipine on cytosolic calcium concentration with fura-2 fluorospectrometry. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:423–7.
Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–86.
Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357.
Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. Identification of Happyhour/MAP4K as alternative Hpo/Mst-like kinases in the Hippo kinase cascade. Dev Cell. 2015;34:642–55.
Gong R, Hong AW, Plouffe SW, Zhao B, Liu G, Yu FX, et al. Opposing roles of conventional and novel PKC isoforms in Hippo-YAP pathway regulation. Cell Res. 2015;25:985–8.
Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–7.
Soh JW, Weinstein IB. Roles of specific isoforms of protein kinase C in the transcriptional control of cyclin D1 and related genes. J Biol Chem. 2003;278:34709–16.
Blobe GC, Stribling DS, Fabbro D, Stabel S, Hannun YA. Protein kinase C beta II specifically binds to and is activated by F-actin. J Biol Chem. 1996;271:15823–30.
Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68.
Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600.
Shao X, Li Q, Mogilner A, Bershadsky AD, Shivashankar GV. Mechanical stimulation induces formin-dependent assembly of a perinuclear actin rim. Proc Natl Acad Sci USA. 2015;112:E2595–2601.
Wales P, Schuberth CE, Aufschnaiter R, Fels J, Garcia-Aguilar I, Janning A, et al. Calcium-mediated actin reset (CaAR) mediates acute cell adaptations. Elife. 2016;5:e19850
Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, et al. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–7.
Liu H, Hughes JD, Rollins S, Chen B, Perkins E. Calcium entry via ORAI1 regulates glioblastoma cell proliferation and apoptosis. Exp Mol Pathol. 2011;91:753–60.
Faulkner JK, McGibney D, Chasseaud LF, Perry JL, Taylor IW. The pharmacokinetics of amlodipine in healthy volunteers after single intravenous and oral doses and after 14 repeated oral doses given once daily. Br J Clin Pharmacol. 1986;22:21–25.
Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008;88:1341–78.
Sun S, Irvine KD. Cellular organization and cytoskeletal regulation of the Hippo signaling network. Trends Cell Biol. 2016;26:694–704.
Mana-Capelli S, Paramasivam M, Dutta S, McCollum D. Angiomotins link F-actin architecture to Hippo pathway signaling. Mol Biol Cell. 2014;25:1676–85.
Li W, Cooper J, Zhou L, Yang C, Erdjument-Bromage H, Zagzag D, et al. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the Hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell. 2014;26:48–60.
Acknowledgements
We thank Thomas Abraham and Wade Edris in the Microscopy Imaging Core Facility, Kang Li in the Molecular and Histopathology Core Facility, and Wesley Raup-Konsavage in the Drug Discovery, Development and Delivery Core Facility of Penn State College of Medicine for technical support and sample analysis. This work was supported by the National Institutes of Health MSTP Grant 5T32GM118294 (to P.P.Y. through PSU), National Institutes of Health Grants R01HL097111 and R01HL123364 (to M.T.), K22 5K22CA190440 (to W.L.), AACR-Aflac, Inc. Career Development Award for Pediatric Cancer Research 14-20-10-LI (to W.L.), and the Four Diamonds Fund for Pediatric Cancer Research (to W.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, Z., Wei, Y., Zhang, L. et al. Induction of store-operated calcium entry (SOCE) suppresses glioblastoma growth by inhibiting the Hippo pathway transcriptional coactivators YAP/TAZ. Oncogene 38, 120–139 (2019). https://doi.org/10.1038/s41388-018-0425-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0425-7
This article is cited by
-
Neuronal Store-Operated Calcium Channels
Molecular Neurobiology (2023)
-
Calcium channel blockers improve the prognosis of patients with intrahepatic cholangiocarcinoma after resection
Journal of Gastroenterology (2022)
-
Structure and function of the N-terminal extension of the formin INF2
Cellular and Molecular Life Sciences (2022)
-
Concerted localization-resets precede YAP-dependent transcription
Nature Communications (2020)
-
Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression
Nature Communications (2020)