Abstract
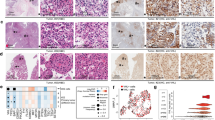
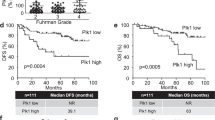
Metastatic clear cell renal cell carcinoma (CCC) remains incurable despite advances in the development of anti-angiogenic targeted therapies and the emergence of immune checkpoint inhibitors. We have previously shown that the sonic hedgehog-Gli signaling pathway is oncogenic in CCC allowing us to identify the developmental Lim1 transcription factor as a Gli target and as a new oncogene in CCC regulating cell proliferation and apoptosis, and promoting tumor growth. In this previous study, preliminary in vitro results also suggested that Lim1 may be implicated in metastatic spread. Here we investigated the potential pro-metastatic role of Lim1 in advanced CCC (1) in vitro using a panel of CCC cell lines expressing or not the von Hippel-Lindau (VHL) tumor suppressor gene either naturally or by gene transfer and (2) ex vivo in 30 CCC metastatic tissues, including lymph nodes, lung, skin, bone, and adrenal metastases, and (3) in vivo, using a metastatic model by intravenous injection of siRNA-transfected cells into Balb/c nude. Our in vitro results reveal that Lim1 knockdown time-dependently decreased CCC cell motility, migration, invasion, and clonogenicity by up to 50% regardless of their VHL status. Investigating the molecular machinery involved in these processes, we identified a large panel of Lim1 targets known to be involved in cell adhesion (paxillin and fibronectin), epithelial-mesenchymal transition (Twist1/2 and snail), invasion (MMP1/2/3/8/9), and metastatic progression (CXCR4, SDF-1, and ANG-1). Importantly, Lim1 was found constitutively expressed in all metastatic tissues. The H-score in metastatic tissues being significantly superior to the score in the corresponding primary tumor tissues (P value = 0.009). Furthermore, we showed that Lim1 silencing decreases pulmonary metastasis development in terms of number and size in the in vivo metastatic model of human CCC. Taken together, these experiments strengthen the potential therapeutic value of Lim1 targeting as a promising novel approach for treating metastatic human CCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386.
Escudier B, Porta C, Bono P, Powles T, Eisen T, Sternberg CN, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Clin Oncol. 2014;32:1412–8.
Posadas EM, Limvorasak S, Figlin RA. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol. 2017;13:496–511.
Mehta K, Patel K, Parikh RA. Immunotherapy in genitourinary malignancies. J Hematol Oncol. 2017;10:95.
Dormoy V, Danilin S, Lindner V, Thomas L, Rothhut S, Coquard C, et al. The sonic hedgehog signaling pathway is reactivated in human renal cell carcinoma and plays orchestral role in tumor growth. Mol Cancer. 2009;8:123.
Sourbier C, Lindner V, Lang H, Agouni A, Schordan E, Danilin S, et al. The phosphoinositide 3-kinase/Akt pathway: a new target in human renal cell carcinoma therapy. Cancer Res. 2006;66:5130–42.
Dormoy V, Béraud C, Lindner V, Thomas L, Coquard C, Barthelmebs M, et al. LIM-class homeobox gene Lim1, a novel oncogene in human renal cell carcinoma. Oncogene. 2011;30:1753–63.
Barnes JD, Crosby JL, Jones CM, Wright CV, Hogan BL. Embryonic expression of Lim-1, the mouse homolog of Xenopus Xlim-1, suggests a role in lateral mesoderm differentiation and neurogenesis. Dev Biol. 1994;161:168–78.
Hukriede NA, Tsang TE, Habas R, Khoo PL, Steiner K, Weeks DL, et al. Conserved requirement of Lim1 function for cell movements during gastrulation. Dev Cell. 2003;4:83–94.
Cheah SS, Kwan KM, Behringer RR. Requirement of LIM domains for LIM1 function in mouse head development. Genesis. 2000;27:12–21.
Zhao Y, Kwan KM, Mailloux CM, Lee WK, Grinberg A, Wurst W, et al. LIM-homeodomain proteins Lhx1 and Lhx5, and their cofactor Ldb1, control Purkinje cell differentiation in the developing cerebellum. Proc Natl Acad Sci USA. 2007;104:13182–6.
Ledig S, Brucker S, Barresi G, Schomburg J, Rall K, Wieacker P. Frame shift mutation of LHX1 is associated with Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome. Hum Reprod. 2012;27:2872–5.
Huang CC, Orvis GD, Kwan KM, Behringer RR. Lhx1 is required in Müllerian duct epithelium for uterine development. Dev Biol. 2014;389:124–36.
Cirio MC, Hui Z, Haldin CE, Cosentino CC, Stuckenholz C, Chen X, et al. Lhx1 is required for specification of the renal progenitor cell field. PLoS ONE. 2011;6:e18858.
Costello I, Nowotschin S, Sun X, Mould AW, Hadjantonakis AK, Bikoff EK, et al. Lhx1 functions together with Otx2, Foxa2, and Ldb1 to govern anterior mesendoderm, node, and midline development. Genes Dev. 2015;29:2108–22.
Ye L, Evans J, Gargett CE. Lim1/LIM1 is expressed in developing and adult mouse and human endometrium. Histochem Cell Biol. 2012;137:527–36.
Varis A, Wolf M, Monni O, Vakkari ML, Kokkola A, Moskaluk C, et al. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res. 2002;62:2625–9.
Sato N, Fukushima N, Maitra A, Matsubayashi H, Yeo CJ, Cameron J, et al. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res. 2003;63:3735–42.
Tong WG, Wierda WG, Lin E, Kuang SQ, Bekele BN, Estrov Z, et al. Genome-wide DNA methylation profiling of chronic lymphocytic leukemia allows identification of epigenetically repressed molecular pathways with clinical impact. Epigenetics. 2010;5:499–508.
Guertl B, Senanayake U, Nusshold E, Leuschner I, Mannweiler S, Ebner B, et al. Lim1, an embryonal transcription factor, is absent in multicystic renal dysplasia, but reactivated in nephroblastomas. Pathobiology. 2011;78:210–9.
Mumert M, Dubuc A, Wu X, Northcott PA, Chin SS, Pedone CA, et al. Functional genomics identifies drivers of medulloblastoma dissemination. Cancer Res. 2012;72:4944–53.
Qu LS, Jin F, Guo YM, Liu TT, Xue RY, Huang XW, et al. Nine susceptibility loci for hepatitis B virus-related hepatocellular carcinoma identified by a pilot two-stage genome-wide association study. Oncol Lett. 2016;11:624–32.
Mikami S, Oya M, Mizuno R, Kosaka T, Ishida M, Kuroda N, et al. Recent advances in renal cell carcinoma from a pathological point of view. Pathol Int. 2016;66:481–90.
Lin TC, Liu YP, Chan YC, Su CY, Lin YF, Hsu SL, et al. Ghrelin promotes renal cell carcinoma metastasis via Snail activation and is associated with poor prognosis. J Pathol. 2015;237:50–61.
Lin YW, Lee LM, Lee WJ, Chu CY, Tan P, Yang YC, et al. Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF-κB DNA-binding activity. J Pineal Res. 2016;60:277–90.
Zhao Z, Liu H, Hou J, Li T, Du X, Zhao X, et al. Tumor protein D52 (TPD52) inhibits growth and metastasis in renal cell carcinoma cells through the PI3K/Akt signaling pathway. Oncol Res. 2017;25:773–9.
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92.
Dormoy V, Jacqmin D, Lang H, Massfelder T. From development to cancer: lessons from the kidney to uncover new therapeutic targets. Anticancer Res. 2012;32:3609–17.
Li H, Yue D, Jin JQ, Woodard GA, Tolani B, Luh TM, et al. Gli promotes epithelial-mesenchymal transition in human lung adenocarcinomas. Oncotarget. 2016;7:80415–25.
Stanton BZ, Peng LF. Small-molecule modulators of the Sonic Hedgehog signaling pathway. Mol Biosyst. 2010;6:44–54.
Sourbier C, Danilin S, Lindner V, Steger J, Rothhut S, Meyer N, et al. Targeting the nuclear factor-kappaB rescue pathway has promising future in human renal cell carcinoma therapy. Cancer Res. 2007;67:11668–76.
Beksac AT, Paulucci DJ, Blum KA, Yadav SS, Sfakianos JP, Badani KK. Heterogeneity in renal cell carcinoma. Urol Oncol. 2017;S1078-1439:30216–8.
Xia Y, Yeddula N, Leblanc M, Ke E, Zhang Y, Oldfield E, et al. Reduced cell proliferation by IKK2 depletion in a mouse lung-cancer model. Nat Cell Biol. 2012;14:257–65.
Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res. 2001;7:3113–9.
Jeong DE, Song HJ, Lim S, Lee SJ, Lim JE, Nam DH, et al. Repurposing the anti-malarial drug artesunate as a novel therapeutic agent for metastatic renal cell carcinoma due to its attenuation of tumor growth, metastasis, and angiogenesis. Oncotarget. 2015;6:33046–64.
Ruan H, Yang H, Wei H, Xiao W, Lou N, Qiu B, et al. Overexpression of SOX4 promotes cell migration and invasion of renal cell carcinoma by inducing epithelial-mesenchymal transition. Int J Oncol. 2017;51:336–46.
Wang Y, Fu D, Su J, Chen Y, Qi C, Sun Y, et al. C1QBP suppresses cell adhesion and metastasis of renal carcinoma cells. Sci Rep. 2017;7:999.
Yerokhin VA, Shabaev VM. Nuclear recoil effect in the lamb shift of light hydrogenlike atoms. Phys Rev Lett. 2015;115:233002.
Liu L, Li Y, Liu S, Duan Q, Chen L, Wu T, et al. Downregulation of miR-193a-3p inhibits cell growth and migration in renal cell carcinoma by targeting PTEN. Tumour Biol. 2017;39:1010428317711951.
Li JK, Chen C, Liu JY, Shi JZ, Liu SP, Liu B, et al. Long noncoding RNA MRCCAT1 promotes metastasis of clear cell renal cell carcinoma via inhibiting NPR3 and activating p38-MAPK signaling. Mol Cancer. 2017;16:111.
Yang H, Huo P, Hu G, Wei B, Kong D, Li H. Identification of gene markers associated with metastasis in clear cell renal cell carcinoma. Oncol Lett. 2017;13:4755–61.
Zhu J, Liang C, Hua Y, Miao C, Zhang J, Xu A, et al. The metastasis suppressor CD82/KAI1 regulates cell migration and invasion via inhibiting TGF-β 1/Smad signaling in renal cell carcinoma. Oncotarget. Oncotarget. 2017;8:51559–68.
Gao Y, Li H, Ma X, Fan Y, Ni D, Zhang Y, et al. KLF6 suppresses metastasis of clear cell renal cell carcinoma via transcriptional repression of E2F1. Cancer Res. 2017;77:330–42.
Scelo G, Purdue MP, Brown KM, Johansson M, Wang Z, Eckel-Passow JE, et al. Genome-wide association study identifies multiple risk loci for renal cell carcinoma. Nat Commun. 2017;8:15724.
Neuberg P, Hamaidi I, Danilin S, Ripoll M, Lindner V, Nothisen M, et al. Polydiacetylenic nanofibers as new siRNA vehicles for in vitro and in vivo delivery. Nanoscale. 2018;10:1587–90.
Delahunt B, Egevad L, Samaratunga H, Varma M, Verrill C, Cheville J, et al. UICC drops the ball in the8th edition TNM staging of urological cancers. Histopathology. 2017;71:5–11.
Béraud C, Dormoy V, Danilin S, Lindner V, Béthry A, Hochane M, et al. Targeting FAK scaffold functions inhibits human renal cell carcinoma growth. Int J Cancer. 2015;137:1549–59.
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9.
Acknowledgements
This study was sponsored by INSERM (recipient TM), the University of Strasbourg (recipient TM), and the Ligue Contre le Cancer (recipient TM). The authors thank Martine MUCKENSTURM, Fabienne REYMANN, and Angélique WERCK, from the Department of Pathology, University Hospital, Strasbourg, for their technical assistance in immunohistochemistry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hamaidi, I., Coquard, C., Danilin, S. et al. The Lim1 oncogene as a new therapeutic target for metastatic human renal cell carcinoma. Oncogene 38, 60–72 (2019). https://doi.org/10.1038/s41388-018-0413-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0413-y
This article is cited by
-
Chemotherapy exacerbates ovarian cancer cell migration and cancer stem cell-like characteristics through GLI1
British Journal of Cancer (2020)
-
The interaction of YBX1 with G3BP1 promotes renal cell carcinoma cell metastasis via YBX1/G3BP1-SPP1- NF-κB signaling axis
Journal of Experimental & Clinical Cancer Research (2019)
-
A panel of Transcription factors identified by data mining can predict the prognosis of head and neck squamous cell carcinoma
Cancer Cell International (2019)