Abstract
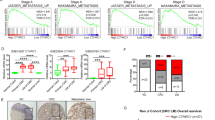
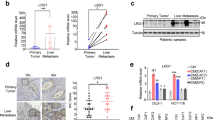
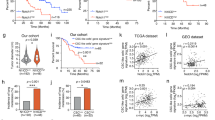
Liver metastases remain a major cause of death from gastrointestinal tract cancers as well as from other malignancies such as breast and lung carcinomas and melanoma. Understanding the underlying biology is essential for the design of effective targeted therapies. We previously reported that collagen IV α1/α2 overexpression in non-metastatic lung carcinoma (M27colIV) cells increased their metastatic ability, specifically to the liver and documented high collagen IV levels in surgical resections of liver metastases from diverse tumor types. Here, we aimed to elucidate the functional relevance of collagen IV to metastatic outgrowth in the liver. Gene expression profiling revealed in M27colIVcells significant increases in the expression of chemokines CCL5 (5.7-fold) and CCL7 (2.6-fold) relative to wild-type cells, and this was validated by qPCR and western blotting. Similarly, in human colon carcinoma KM12C and KM12SM cells with divergent liver-colonizing potentials, CCL7 and CCL5 production correlated with type IV collagen expression and the metastatic phenotype. CCL7 silencing by short hairpin RNA (shRNA) reduced experimental liver metastasis in both cell types, whereas CCL5 silencing reduced metastasis of M27colIV cells, implicating these cytokines in metastatic expansion in the liver. Subsequent functional analyses implicated both MEK/ERK and PI3K signaling upstream of CCL7 upregulation and identified CCL7 (but not CCL5) as a critical migration/invasion factor, acting via the chemokine receptor CCR3. Chemokine CCL5 was identified as a regulator of the T-cell immune response in the liver. Loss of CCL7 in KM12SM cells was also associated with altered E-cadherin and reduced vimentin and Snail expression, implicating it in epithelial-to-mesenchymal transition in these cells. Moreover, in clinical specimens of colon cancer liver metastases analyzed by immunohistochemistry, CCL5 and CCL7 levels paralleled those of collagen IV. The results identify the chemokines CCL5 and CCL7 as type IV collagen-regulated genes that promote liver metastasis by distinct and complementary mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101.
Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8.
Fidler IJ, Poste G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008;9:808.
Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5:402–18.
Burnier JV, Wang N, Michel RP, Hassanain M, Li S, Lu Y, et al. Type IV collagen-initiated signals provide survival and growth cues required for liver metastasis. Oncogene. 2011;30:3766–83.
Akram IG, Georges R, Hielscher T, Adwan H, Berger MR. The chemokines CCR1 and CCRL2 have a role in colorectal cancer liver metastasis. Tumour Biol. 2016;37:2461–71.
Struyf S, Menten P, Lenaerts JP, Put W, D’Haese A, De Clercq E, et al. Diverging binding capacities of natural LD78beta isoforms of macrophage inflammatory protein-1alpha to the CC chemokine receptors 1, 3 and 5 affect their anti-HIV-1 activity and chemotactic potencies for neutrophils and eosinophils. Eur J Immunol. 2001;31:2170–8.
Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–85.
Chang LY, Lin YC, Mahalingam J, Huang CT, Chen TW, Kang CW, et al. Tumor-derived chemokine CCL5 enhances TGF-beta-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res. 2012;72:1092–102.
Luo J, Lee SO, Cui Y, Yang R, Li L, Chang C. Infiltrating bone marrow mesenchymal stem cells (BM-MSCs) increase prostate cancer cell invasion via altering the CCL5/HIF2alpha/androgen receptor signals. Oncotarget. 2015;6:27555–65.
Cambien B, Richard-Fiardo P, Karimdjee BF, Martini V, Ferrua B, Pitard B, et al. CCL5 neutralization restricts cancer growth and potentiates the targeting of PDGFRbeta in colorectal carcinoma. PLoS ONE. 2011;6:e28842.
Mrowietz U, Schwenk U, Maune S, Bartels J, Kupper M, Fichtner I, et al. The chemokine RANTES is secreted by human melanoma cells and is associated with enhanced tumour formation in nude mice. Br J Cancer. 1999;79:1025–31.
Stormes KA, Lemken CA, Lepre JV, Marinucci MN, Kurt RA. Inhibition of metastasis by inhibition of tumor-derived CCL5. Breast Cancer Res Treat. 2005;89:209–12.
Lee YS, Kim SY, Song SJ, Hong HK, Lee Y, Oh BY, et al. Crosstalk between CCL7 and CCR3 promotes metastasis of colon cancer cells via ERK-JNK signaling pathways. Oncotarget. 2016;7:36842–53.
Jung DW, Che ZM, Kim J, Kim K, Kim KY, Williams D. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. Int J Cancer. 2010;127:332–44.
Cho YB, Lee WY, Choi SJ, Kim J, Hong HK, Kim SH, et al. CC chemokine ligand 7 expression in liver metastasis of colorectal cancer. Oncol Rep. 2012;28:689–94.
Hirai H, Fujishita T, Kurimoto K, Miyachi H, Kitano S, Inamoto S, et al. CCR1-mediated accumulation of myeloid cells in the liver microenvironment promoting mouse colon cancer metastasis. Clin Exp Metastasis. 2014;31:977–89.
Hoyer M, Erichsen R, Gandrup P, Norgaard M, Jacobsen JB. Survival in patients with synchronous liver metastases in central and northern Denmark, 1998 to 2009. Clin Epidemiol. 2011;3(Suppl 1):11–7.
Turdean S, Gurzu S, Turcu M, Voidăzan S, Sin A. Liver Metastases: Incidence and Clinicopathological Data. Acta Med Marisiensis. 2012;58:254–8.
Kitadai Y, Bucana CD, Ellis LM, Anzai H, Tahara E, Fidler IJ. In situ mRNA hybridization technique for analysis of metastasis-related genes in human colon carcinoma cells. Am J Pathol. 1995;147:1238–47.
Zhang D, Bar-Eli M, Meloche S, Brodt P. Dual regulation of MMP-2 expression by the type 1 insulin-like growth factor receptor: the phosphatidylinositol 3-kinase/Akt and Raf/ERK pathways transmit opposing signals. J Biol Chem. 2004;279:19683–90.
Zhang D, Brodt P. Type 1 insulin-like growth factor regulates MT1-MMP synthesis and tumor invasion via PI 3-kinase/Akt signaling. Oncogene . 2003;22:974–82.
Nystrom H, Tavelin B, Bjorklund M, Naredi P, Sund M. Improved tumour marker sensitivity in detecting colorectal liver metastases by combined type IV collagen and CEA measurement. Tumour Biol. 2015;36:9839–47.
Shen X, Yue M, Meng F, Zhu J, Zhu X, Jiang Y. Microarray analysis of differentially-expressed genes and linker genes associated with the molecular mechanism of colorectal cancer. Oncol Lett. 2016;12:3250–8.
Vellinga TT, den Uil S, Rinkes IH, Marvin D, Ponsioen B, Alvarez-Varela A, et al. Collagen-rich stroma in aggressive colon tumors induces mesenchymal gene expression and tumor cell invasion. Oncogene. 2016;35:5263–71.
Yoshimura K, Meckel KF, Laird LS, Chia CY, Park JJ, Olino KL, et al. Integrin alpha2 mediates selective metastasis to the liver. Cancer Res. 2009;69:7320–8.
Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–51.
Moreno-Layseca P, Streuli CH. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 2014;34:144–53.
Bai D, Ueno L, Vogt PK. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer. 2009;125:2863–70.
Brunner PM, Glitzner E, Reininger B, Klein I, Stary G, Mildner M, et al. CCL7 contributes to the TNF-alpha-dependent inflammation of lesional psoriatic skin. Exp Dermatol. 2015;24:522–8.
Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res. 2011;9:133–48.
Li S, Pinard M, Wang Y, Yang L, Lin R, Hiscott J, et al. Crosstalk between the TNF and IGF pathways enhances NF-kappaB activation and signaling in cancer cells. Growth Horm IGF Res. 2015;25:253–61.
Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47.
Su B, Zhao W, Shi B, Zhang Z, Yu X, Xie F, et al. Let-7d suppresses growth, metastasis, and tumor macrophage infiltration in renal cell carcinoma by targeting COL3A1 and CCL7. Mol Cancer. 2014;13:206.
Brodt P. Role of the microenvironment in liver metastasis: from pre- to prometastatic niches. Clin Cancer Res. 2016;22:5971–82.
Goos JA, Coupe VM, van de Wiel MA, Diosdado B, Delis-Van Diemen PM, Hiemstra AC, et al. A prognostic classifier for patients with colorectal cancer liver metastasis, based on AURKA, PTGS2 and MMP9. Oncotarget. 2016;7:2123–34.
Yamada M, Ichikawa Y, Yamagishi S, Momiyama N, Ota M, Fujii S, et al. Amphiregulin is a promising prognostic marker for liver metastases of colorectal cancer. Clin Cancer Res. 2008;14:2351–6.
Eefsen RL, Van den Eynden GG, Hoyer-Hansen G, Brodt P, Laerum OD, Vermeulen PB, et al. Histopathological growth pattern, proteolysis and angiogenesis in chemonaive patients resected for multiple colorectal liver metastases. J Oncol. 2012;2012:907971.
Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22:1294–302.
Van den Eynden GG, Bird NC, Dirix LY, Eefsen RL, Gao ZH, Hoyer-Hansen G, et al. Tumor stromal phenotypes define VEGF sensitivity--letter. Clin Cancer Res. 2014;20:5140.
Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Hoyer-Hansen G, et al. The multifaceted role of the microenvironment in liver metastasis: biology and clinical implications. Cancer Res. 2013;73:2031–43.
Proudfoot AE, Bonvin P, Power CA. Targeting chemokines: pathogens can, why can’t we? Cytokine. 2015;74:259–67.
Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16:829–42.
Brodt P, Fallavollita L, Khatib AM, Samani AA, Zhang D. Cooperative regulation of the invasive and metastatic phenotypes by different domains of the type I insulin-like growth factor receptor beta subunit. J Biol Chem. 2001;276:33608–15.
Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48:6863–71.
Samani AA, Chevet E, Fallavollita L, Galipeau J, Brodt P. Loss of tumorigenicity and metastatic potential in carcinoma cells expressing the extracellular domain of the type 1 insulin-like growth factor receptor. Cancer Res. 2004;64:3380–5.
Acknowledgements
We thank Dr. Julia Burnier for the initial analysis of the microarray data, Ms. Anette Berglund for skillful technical assistance with immunohistochemistry, and the laboratory of Dr. Jean-Jacques Lebrun for help and advice. This work was supported mainly by grants MOP 80201 from the Canadian Institute for Health Research and a PSR-SIIRI-843 grant from the Québec Ministère de l'Économie, de l’Innovation et des Exportations (to P.B.) and also by grants from the Swedish Research Council and Västerbotten County Council (to H.N.). R.F.R. was supported by the Henry R. Shibata fellowship from the Cedars Cancer Institute. R.F.R. and G.V. were supported by MITACS internships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vaniotis, G., Rayes, R.F., Qi, S. et al. Collagen IV-conveyed signals can regulate chemokine production and promote liver metastasis. Oncogene 37, 3790–3805 (2018). https://doi.org/10.1038/s41388-018-0242-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0242-z
This article is cited by
-
Cell-cell communication characteristics in breast cancer metastasis
Cell Communication and Signaling (2024)
-
PARG suppresses tumorigenesis and downregulates genes controlling angiogenesis, inflammatory response, and immune cell recruitment
BMC Cancer (2022)
-
Characterization of TGFβ-associated molecular features and drug responses in gastrointestinal adenocarcinoma
BMC Gastroenterology (2021)
-
Cancer-associated fibroblasts promote oral squamous cell carcinoma progression through LOX-mediated matrix stiffness
Journal of Translational Medicine (2021)
-
Liver metastases
Nature Reviews Disease Primers (2021)