Abstract
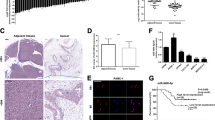
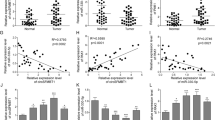
Migration and invasion inhibitory protein (MIIP) is recently identified as an inhibitor in tumor development. However, the regulatory mechanism and biological contributions of MIIP in pancreatic cancer (PC) have been not elucidated. In this study, we demonstrated a negative feedback of MIIP and hypoxia-induced factor-1α (HIF-1α), which was mediated by a hypoxia-induced microRNA. Compared with paracarcinoma tissues, MIIP was downregulated in PC tissues. Overexpression of MIIP significantly impeded the proliferation and invasion of PC cells both in vitro and in mouse xenograft models. We further verified MIIP was downregulated under hypoxia in a HIF-1α-mediated manner. Interestingly, although MIIP promoter containing two putative hypoxia response elements (HREs), the chromatin immunoprecipitation (ChIP) and luciferase reporter assays did not support an active interaction between HIF-1α and MIIP promoter. Meanwhile, microRNA array revealed a hypoxia-induced microRNA, miR-646, impaired stability of MIIP mRNA and consequently inhibited its expression by targeting the coding sequence (CDS). Coincidently, knockdown of miR-646 significantly repressed proliferation and invasion ability of PC cells both in vitro and in vivo by upregulating MIIP expression. Besides, ChIP and luciferase reporter assays further validated that HIF-1α activated transcription of miR-646 in hypoxia condition. Therefore, these results suggested HIF-1α indirectly regulated MIIP expression in post-transcriptional level through upregulating miR-646 transcription. Conversely, our results further revealed that MIIP suppressed deacetylase ability of histone deacetylase 6 (HDAC6) to promote the acetylation and degradation of HIF-1α, by which impairing HIF-1α accumulation. What is more, a specific relationship between downregulated MIIP and upregulated miR-646 expression was validated in PC samples. Moreover, the dysregulated miR-646 and MIIP expression was correlated with advanced tumor stage, lymphatic invasion, metastasis and shorter overall survival in PC patients. Together, our results highlight that the reciprocal loop of HIF-1α/miR-646/MIIP might be implemented as an applicable target for pancreatic cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Wang Y, Wen J, Zhang W. MIIP, a cytoskeleton regulator that blocks cell migration and invasion, delays mitosis, and suppresses tumorogenesis. Curr Protein Pept Sci. 2011;12:68–73.
Sun Y, Ji P, Chen T, Zhou X, Yang D, Guo Y, et al. MIIP haploinsufficiency induces chromosomal instability and promotes tumour progression in colorectal cancer. J Pathol. 2016;241:67–79.
Jing W, Fu J, Ling Y, Wei Z. MIIP accelerates epidermal growth factor receptor protein turnover and attenuates proliferation in non-small cell lung cancer. Oncotarget. 2011;7:9118–34.
Wang Y, Hu L, Ping J, Fei T, Tian W, Liu Y, et al. MIIP remodels Rac1-mediated cytoskeleton structure in suppression of endometrial cancer metastasis. J Hematol Oncol. 2016;9:112.
Song F, Zhang L, Ji P, Zheng H, Zhao Y, Zhang W, et al. Altered expression and loss of heterozygosity of the migration and invasion inhibitory protein (MIIP) gene in breast cancer. Oncol Rep. 2015;33:2771–8.
Ji P, Smith SM, Wang Y, Jiang R, Song SW, Li B, et al. Inhibition of gliomagenesis and attenuation of mitotic transition by MIIP. Oncogene. 2010;29:3501–8.
Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437.
Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastas-- Rev. 2007;26:225.
Pothula SP, Xu Z, Goldstein D, Pirola RC, Wilson JS, Apte MV. Key role of pancreatic stellate cells in pancreatic cancer. Cancer Lett. 2015;24:232–8.
Gomezroman N, Sahasrabudhe NM, Mcgregor F, Chalmers AJ, Cassidy J, Plumb J. Hypoxia-inducible factor 1 alpha is required for the tumourigenic and aggressive phenotype associated with Rab25 expression in ovarian cancer. Oncotarget. 2016;7:22650–64.
Wang X, Ren H, Zhao T, Ma W, Dong J, Zhang S, et al. Single nucleotide polymorphism in the microRNA-199a binding site of HIF1A gene is associated with pancreatic ductal adenocarcinoma risk and worse clinical outcomes. Oncotarget. 2016;7:13717–29.
Kong X, Lin Z, Liang D, Fath D, Sang N, Caro J. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha. Mol Cell Biol. 2006;26:2019.
Qian DZ, Kachhap SK, Collis SJ, Verheul HM, Carducci MA, Atadja P, et al. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res. 2006;66:8814–21.
Zhang D, Li J, Costa M, Gao J, Huang C. JNK1 mediates degradation HIF-1alpha by a VHL-independent mechanism that involves the chaperones Hsp90/Hsp70. Cancer Res. 2010;70:813.
Wen W, Ding J, Sun W, Wu K, Ning B, Gong W, et al. Suppression of cyclin D1 by hypoxia-inducible factor-1 via direct mechanism inhibits the proliferation and 5-fluorouracil-induced apoptosis of A549 cells. Cancer Res. 2010;70:2010.
Lee KJ, Lee KY, Lee YM. Downregulation of a tumor suppressor RECK by hypoxia through recruitment of HDAC1 and HIF-1alpha to reverse HRE site in the promoter. Biochim Biophys Acta. 2010;1803:608.
Shriram N, Chan SY, Joseph L. Hypoxia: a master regulator of microRNA biogenesis and activity. Free Radic Biol Med. 2013;64:20–30.
Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–67.
Fasanaro P, D’Alessandra Y, Di SV, Melchionna R, Romani S, Pompilio G, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand ephrin-A3. J Biol Chem. 2008;283:15878–83.
Zhang D, Shi Z, Li M, Mi J. Hypoxia-induced miR-424 decreases tumor sensitivity to chemotherapy by inhibiting apoptosis. Cell Death Dis. 2014;5:e1301.
Wu C, So J, Davisdusenbery BN, Qi HH, Bloch DB, Shi Y, et al. Hypoxia potentiates microRNA-mediated gene silencing through posttranslational modification of argonaute2. Mol Cell Biol. 2011;31:4760–74.
Chen Z, Li YH, Huang P, Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene. 2010;29:4362.
Loscalzo J. The cellular response to hypoxia: tuning the system with microRNAs. J Clin Invest. 2010;120:3815.
Mace TA, Collins AL, Wojcik SE, Croce CM, Lesinski GB, Bloomston M. Hypoxia induces the overexpression of microRNA-21 in pancreatic cancer cells. J Surg Res. 2013;184:855.
Wu Q, Liu HO, Liu YD, Liu WS, Pan D, Zhang WJ, et al. Decreased expression of hepatocyte nuclear factor 4α (Hnf4α)/microRNA-122 (miR-122) axis in hepatitis B virus-associated hepatocellular carcinoma enhances potential oncogenic GALNT10 protein activity. J Biol Chem. 2015;290:1170–85.
Jones MF, Hara T, Francis P, Li XL, Bilke S, Zhu Y, et al. The CDX1-microRNA-215 axis regulates colorectal cancer stem cell differentiation. Proc Natl Acad Sci USA. 2015;112:1550–8.
Wu Y, Song S. IIp45 inhibits cell migration through inhibition of HDAC6. J Biol Chem. 2010;285:3554–60.
Wang Y, Ji P, Yang D, Hu L, Cogdell D, Broaddus R, et al. Suppression of endometrial cancer cell migration, invasion, and colony formation by the putative tumor suppressor gene MIIP. Cancer Res. 2011;70:423–423.
Ying Q, Liang L, Guo W, Zha R, Tian Q, Huang S, et al. Hypoxia‐inducible MicroRNA‐210 augments the metastatic potential of tumor cells by targeting vacuole membrane protein 1 in hepatocellular carcinoma. Hepatology. 2011;54:2064–75.
Zhang Z, Sun H, Dai H, Walsh RM, Imakura M, Schelter J, et al. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle. 2009;8:2756.
Vaiman D. Genes, epigenetics and miRNA regulation in the placenta. Placenta. 2017;52:127–33.
Hausser J, Syed AP, Bilen B, Zavolan M. Analysis of CDS-located miRNA target sites suggests that they can effectively inhibit translation. Genome Res. 2013;23:604.
Reczko M, Maragkakis M, Alexiou P, Grosse I, Hatzigeorgiou AG. Functional microRNA targets in protein coding sequences. Bioinformatics. 2012;28:771.
Duursma AM, Kedde M, Schrier M, Sage CL, Agami R. miR-148 targets human DNMT3b protein coding region. Rna-a Publ Rna Soc. 2008;14:872.
Zhou H, Rigoutsos I. MiR-103a-3p targets the 5’ UTR of GPRC5A in pancreatic cells. RNA (New Y, NY, USA). 2014;20:1431.
Liu G, Zhang R, Xu J, Wu CI, Lu X. Functional conservation of both CDS- and 3’-UTR-located microRNA binding sites between species. Mol Biol Evol. 2015;32:623.
Hui ABY, Shi W, Boutros PC, Miller N, Pintilie M, Fyles T, et al. Robust global micro-RNA profiling with formalin-fixed paraffin-embedded breast cancer tissues. Lab Invest. 2009;89:597–606.
Wang R, Zhang J, Jiang W, Ma Y, Li W, Jin B, et al. Association between a variant in microRNA-646 and the susceptibility to hepatocellular carcinoma in a large-scale population. Sci World J. 2014;2014:312704.
Li W, Liu M, Feng Y, Xu YF, Huang YF, Che JP, et al. Downregulated miR-646 in clear cell renal carcinoma correlated with tumour metastasis by targeting the nin one binding protein (NOB1). Br J Cancer. 2011;111:1188–1200.
Azam AT, Bahador R, Hesarikia H, Shakeri M, Yeganeh A. Downregulation of microRNA-217 and microRNA-646 acts as potential predictor biomarkers in progression, metastasis, and unfavorable prognosis of human osteosarcoma. Tumor Biol. 2016;37:5769–73.
Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915.
Sermeus A, Michiels C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis. 2011;2:e164.
Zhu S, Zhou HY, Deng SC, Deng SJ, He C, Li X, et al. ASIC1 and ASIC3 contribute to acidity-induced EMT of pancreatic cancer through activating Ca2+/RhoA pathway. Cell Death Dis. 2017;8:e2806.
Deng S, Xiang L, Yi N, Shuai Z, Yan J, Deng S, et al. MiR-652 inhibits acidic microenvironment-induced epithelial-mesenchymal transition of pancreatic cancer cells by targeting ZEB1. Oncotarget. 2015;6:39661–75.
Xiang L, Deng SJ, Shuai Z, Yan J, Cui SP, Chen JY, et al. Hypoxia-induced lncRNA-NUTF2P3-001 contributes to tumorigenesis of pancreatic cancer by derepressing the miR-3923/KRAS pathway. Oncotarget. 2016;7:6000–14.
Liu Y, Li X, Zhu S, Zhang J, Yang M, Qin Q, et al. Ectopic expression of miR-494 inhibited the proliferation, invasion and chemoresistance of pancreatic cancer by regulating SIRT1 and c-Myc. Gene Ther. 2015;22:729.
Zhao G, Zhang JG, Liu Y, Qin Q, Wang B, Tian K, et al. miR-148b functions as a tumor suppressor in pancreatic cancer by targeting AMPKα1. Mol Cancer Ther. 2013;12:83–93.
Chen J, Bai M, Ning C, Xie B, Zhang J, Liao H, et al. Gankyrin facilitates follicle-stimulating hormone-driven ovarian cancer cell proliferation through the PI3K/AKT/HIF-1α/cyclin D1 pathway. Oncogene. 2016;35:2506.
Krzywinski M, Altman N. Points of significance: power and sample size. Nat Methods. 2013;10:1139–40.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (no. 81672406, no. 81372666, no. 30972900, and no. 81502076).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Niu, Y., Jin, Y., Deng, SC. et al. MiRNA-646-mediated reciprocal repression between HIF-1α and MIIP contributes to tumorigenesis of pancreatic cancer. Oncogene 37, 1743–1758 (2018). https://doi.org/10.1038/s41388-017-0082-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-017-0082-2
This article is cited by
-
Molecular profile of metastasis, cell plasticity and EMT in pancreatic cancer: a pre-clinical connection to aggressiveness and drug resistance
Cancer and Metastasis Reviews (2024)
-
Nanoparticles loaded with circ_0086375 for suppressing the tumorigenesis of pancreatic cancer by targeting the miR-646/SLC4A4 axis
Clinical & Experimental Metastasis (2023)
-
CircRNA circTIAM1 promotes papillary thyroid cancer progression through the miR-646/HNRNPA1 signaling pathway
Cell Death Discovery (2022)
-
MIIP functions as a novel ligand for ITGB3 to inhibit angiogenesis and tumorigenesis of triple-negative breast cancer
Cell Death & Disease (2022)
-
Targeting hypoxic tumor microenvironment in pancreatic cancer
Journal of Hematology & Oncology (2021)