Abstract
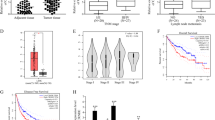
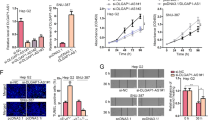
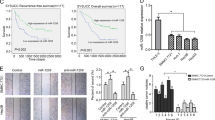
Emerging evidence indicates that the long noncoding RNAs extensively participate in cancer progression. Nevertheless, the molecular pathogenesis of how these lncRNAs regulate tumorigenesis has not been fully elucidated especially in hepatocellular carcinoma (HCC). Here, we sought to define the role of a novel lncRNA named lncRNA-NEF in modulating epithelial to mesenchymal transition (EMT) in HCC. It was found that the lncRNA-NEF was transcriptionally activated by EMT suppressor FOXA2 and frequently downregulated in HCC cell lines as well as clinical specimens. Although enhanced expression of lncRNA-NEF did not affect tumor cell growth, ectopic expression of lncRNA-NEF significantly suppressed EMT program and cell migration. Animal studies validated that lncRNA-NEF alleviated in vivo tumor metastasis and protected mice from tumor-induced mortality. Interestingly, we verified that lncRNA-NEF acted as a novel activator of its neighbor gene FOXA2, which formed a positive feedback loop. Subsequent studies revealed that lncRNA-NEF physically interacted with β-catenin to increase the binding of GSK3β with β-catenin and therefore promoted the inhibitory phosphorylation of β-catenin, leading to the suppression on Wnt/β-catenin signaling and activation of FOXA2 expression. Hence, our findings illustrated a novel feedback loop including FOXA2 and its neighboring gene lncRNA-NEF, which might provide mechanistic insights into the metastatic progress of HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Qi W, Liang W, Jiang H, Miuyee Waye M. The function of miRNA in hepatic cancer stem cell. BioMed Res Int. 2013;2013:358902.
Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093–107.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Rossi L, Zoratto F, Papa A, Iodice F, Minozzi M, Frati L, et al. Current approach in the treatment of hepatocellular carcinoma. World J Gastrointest Oncol. 2010;2:348–59.
De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110.
Nieto MA, Cano A. The epithelial-mesenchymal transition under control: global programs to regulate epithelial plasticity. Semin Cancer Biol. 2012;22:361–8.
Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464–74.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Bba-Rev Cancer. 2009;1796:75–90.
Tang Y, Shu G, Yuan X, Jing N, Song J. FOXA2 functions as a suppressor of tumor metastasis by inhibition of epithelial-to-mesenchymal transition in human lung cancers. Cell Res. 2011;21:316–26.
Wang D, Zhu ZZ, Jiang HM, Zhu JY, Cong WM, Wen BJ, et al. Multiple genes identified as targets for 20q13.12-13.33 gain contributing to unfavorable clinical outcomes in patients with hepatocellular carcinoma. Hepatol Int. 2015;9:438–46.
Akagi T, Luong QT, Gui D, Said J, Selektar J, Yung A, et al. Induction of sodium iodide symporter gene and molecular characterisation of HNF3 beta/FoxA2, TTF-1 and C/EBP beta in thyroid carcinoma cells. Br J Cancer. 2008;99:781–8.
Liu M, Lee DF, Chen CT, Yen CJ, Li LY, Lee HJ, et al. IKKalpha activation of NOTCH links tumorigenesis via FOXA2 suppression. Mol Cell. 2012;45:171–84.
Wang J, Zhu CP, Hu PF, Qian H, Ning BF, Zhang Q, et al. FOXA2 suppresses the metastasis of hepatocellular carcinoma partially through matrix metalloproteinase-9 inhibition. Carcinogenesis. 2014;35:2576–83.
Song Y, Washington MK, Crawford HC. Loss of FOXA1/2 is essential for the epithelial-to-mesenchymal transition in pancreatic cancer. Cancer Res. 2010;70:2115–25.
Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–41.
Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–7.
Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci USA. 2009;106:11667–72.
Richards EJ, Zhang G, Li ZP, Permuth-Wey J, Challa S, Li YJ, et al. Long Non-coding RNAs (LncRNA) regulated by transforming growth factor (TGF) β: LncRNA-hit-mediated TGFβ-induced epithelial to mesenchymal transition in mammary epithelia. J Biol Chem. 2015;290:6857–67.
Liang WC, Fu WM, Wong CW, Wang Y, Wang WM, Hu GX, et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6:22513–25.
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–81.
Lai F, Orom UA, Cesaroni M, Beringer M, Taatjes DJ, Blobel GA, et al. Activating RNAs associate with mediator to enhance chromatin architecture and transcription. Nature. 2013;494:497–501.
Orom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58.
Melo CA, Drost J, Wijchers PJ, van de Werken H, de Wit E, Vrielink JAFO, et al. eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol Cell. 2013;49:524–35.
Vance KW, Sansom SN, Lee S, Chalei V, Kong LS, Cooper SE, et al. The long non-coding RNA Paupar regulates the expression of both local and distal genes. EMBO J. 2014;33:296–311.
Jiang W, Liu YT, Liu R, Zhang K, Zhang Y. The lncRNA DEANR1 facilitates human endoderm differentiation by activating FOXA2 expression. Cell Rep. 2015;11:137–48.
Ng SY, Bogu GK, Soh BS, Stanton LW. The long noncoding RNA RMST interacts with SOX2 to regulate neurogenesis. Mol Cell. 2013;51:349–59.
Lee HK, Jeong S. Beta-Catenin stabilizes cyclooxygenase-2 mRNA by interacting with AU-rich elements of 3’-UTR. Nucleic Acids Res. 2006;34:5705–14.
Mucenski ML, Nation JM, Thitoff AR, Besnard V, Xu Y, Wert SE, et al. Beta-Catenin regulates differentiation of respiratory epithelial cells in vivo. Am J Physiol. 2005;289:L971–9.
Simons BW, Hurley PJ, Huang ZH, Ross AE, Miller R, Marchionni L, et al. Wnt signaling though beta-catenin is required for prostate lineage specification. Dev Biol. 2012;371:246–55.
Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ. Wnt/beta-catenin activation promotes prostate tumor progression in a mouse model. Oncogene. 2011;30:1868–79.
Xiang C, Wang JC, Kou XC, Chen XB, Qin ZY, Jiang Y, et al. Pulmonary expression of CYP2A13 and ABCB1 is regulated by FOXA2, and their genetic interaction is associated with lung cancer. FASEB J. 2015;29:1986–98.
Liang WC, Fu WM, Wang YB, Sun YX, Xu LL, Wong CW, et al. H19 activates Wnt signaling and promotes osteoblast differentiation by functioning as a competing endogenousRNA. Sci Rep. 2016;6:20121.
Liang WC, Wang Y, Xiao LJ, Wang YB, Fu WM, Wang WM, et al. Identification of miRNAs that specifically target tumor suppressive KLF6-FL rather than oncogenic KLF6-SV1 isoform. RNA Biol. 2014;11:845–54.
Liang WC, Wang Y, Liang PP, Pan XQ, Fu WM, Yeung VS, et al. MiR-25 suppresses 3T3-L1 adipogenesis by directly targeting KLF4 and C/EBPalpha. J Cell Biochem. 2015;116:2658–66.
Liang WC, Wang Y, Wan DC, Yeung VS, Waye MM. Characterization of miR-210 in 3T3-L1 adipogenesis. J Cell Biochem. 2013;114:2699–707.
Fu WM, Lu YF, Hu BG, Liang WC, Zhu X, Yang HD, Li G, Zhang JF. Long noncoding RNA Hotair mediated angiogenesis in nasopharyngeal carcinoma by direct and indirect signaling. Oncotarget. 2015;7:4712–23.
Fu WM, Zhu X, Wang WM, Lu YF, Hu BG, Wang H, et al. Hotair mediates hepatocarcinogenesis through suppressing miRNA-218 expression and activating P14 and P16 signaling. J Hepatol. 2015;63:886–95.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81773066 to W.F. and 81772404 to J.Z.).
Author contributions
W.L. and J.Z. designed the studies. W.L., J.R., and C.W. conducted the experiments. S.C. provided technical support. W.L. drafted the manuscript. W.F. and J.Z. revised the draft. M.M.W. reviewed the data and provided experimental materials.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Liang, WC., Ren, JL., Wong, CW. et al. LncRNA-NEF antagonized epithelial to mesenchymal transition and cancer metastasis via cis-regulating FOXA2 and inactivating Wnt/β-catenin signaling. Oncogene 37, 1445–1456 (2018). https://doi.org/10.1038/s41388-017-0041-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-017-0041-y
This article is cited by
-
FOXA1 and FOXA2: the regulatory mechanisms and therapeutic implications in cancer
Cell Death Discovery (2024)
-
Genome-wide differential expression profiling of long non-coding RNAs in FOXA2 knockout iPSC-derived pancreatic cells
Cell Communication and Signaling (2023)
-
LncRNA-NEF suppressed oxaliplatin resistance and epithelial-mesenchymal transition in colorectal cancer through epigenetically inactivating MEK/ERK signaling
Cancer Gene Therapy (2023)
-
Circular RNA circGLIS3 promotes bladder cancer proliferation via the miR-1273f/SKP1/Cyclin D1 axis
Cell Biology and Toxicology (2022)
-
The functions of lncRNAs in the HPV-negative cervical cancer compared with HPV-positive cervical cancer
Apoptosis (2022)