Abstract
Cocaine abuse continues to be a serious health problem worldwide. Despite intense research, there is still no FDA-approved medication to treat cocaine use disorder (CUD). In this report, we explored the potential utility of beta-caryophyllene (BCP), an FDA-approved food additive for the treatment of CUD. We found that BCP, when administered intraperitoneally or intragastrically, dose-dependently attenuated cocaine self-administration, cocaine-conditioned place preference, and cocaine-primed reinstatement of drug seeking in rats. In contrast, BCP failed to alter food self-administration or cocaine-induced hyperactivity. It also failed to maintain self-administration in a drug substitution test, suggesting that BCP has no abuse potential. BCP was previously reported to be a selective CB2 receptor agonist. Unexpectedly, pharmacological blockade or genetic deletion of CB1, CB2, or GPR55 receptors in gene-knockout mice failed to alter BCP’s action against cocaine self-administration, suggesting the involvement of non-CB1, non-CB2, and non-GPR55 receptor mechanisms. Furthermore, pharmacological blockade of μ opioid receptor or Toll-like receptors complex failed to alter, while blockade of peroxisome proliferator-activated receptors (PPARα, PPARγ) reversed BCP-induced reduction in cocaine self-administration, suggesting the involvement of PPARα and PPARγ in BCP’s action. Finally, we used electrical and optogenetic intracranial self-stimulation (eICSS, oICSS) paradigms to study the underlying neural substrate mechanisms. We found that BCP is more effective in attenuation of cocaine-enhanced oICSS than eICSS, the former driven by optical activation of midbrain dopamine neurons in DAT-cre mice. These findings indicate that BCP may be useful for the treatment of CUD, likely by stimulation of PPARα and PPARγ in the mesolimbic system.
Similar content being viewed by others
Introduction
Cocaine is a commonly used psychostimulant worldwide [1]. Despite many years of extensive research, there is still no effective medication to treat cocaine use disorder (CUD). One of the recent medication strategies has been focused on the endocannabinoid system because of its critical role in reward and addiction [2, 3], including cocaine addiction-related behaviors [4,5,6,7,8]. Previous studies have been focused on CB1 receptor (CB1R) antagonists or inverse agonists for their potential utility for the treatment of CUD. The results are mixed. Some studies indicate that CB1R antagonists are effective in decreasing cocaine-conditioned place preference (CPP) [9,10,11], cocaine self-administration [7, 12], and reinstatement of cocaine-seeking behavior [13,14,15], while others indicate a lack of effect on cocaine-self-administration [16,17,18,19,20,21]. Although mice lacking CB1Rs can acquire cocaine CPP [22, 23] and self-administer cocaine [8, 17], they show a significant reduction in cocaine self-administration [8] and the accumbal DA response to cocaine [24]. Likewise, stimulation of CB2Rs or overexpression of CB2Rs in the brain also decreases cocaine self-administration in mice [12, 25]. Despite the promising results from preclinical studies, clinical trials with selective CB1R antagonists failed due to significant adverse effects such as depression and suicidal tendencies [26]. Therefore, many researchers have shifted their interests from CB1Rs to other targets of the endocannabinoid system, such as CB2 receptor [3, 27] and non-psychotomimetic phytocannabinoids [2].
Beta-caryophyllene (BCP) is an FDA-approved food additive that is present in high concentrations across a variety of plants and herbs including black pepper, cloves, rosemary, and cannabis [28, 29]. In 2008, BCP was identified as a selective CB2R agonist [30] with significant anti-inflammatory effects [30,31,32]. Since then, copious therapeutic effects of BCP have been reported including analgesic, anti-depressive, anxiolytic, and neuroprotective [29, 32,33,34,35,36,37], making it a viable candidate in the treatment of CNS disorders. However, little is known about its therapeutic potential in the treatment of substance use disorders.
We recently reported that systemic administration of BCP reduced nicotine self-administration in rats, wild type mice, and at high doses, also in CB2-knockout mice [38]. In addition, pretreatment with AM630 (a CB2R antagonist), but not AM251 (a CB1R antagonist), blocked BCP’s action against nicotine self-administration produced by low doses of BCP (10, 25 mg/kg); both outcomes suggest the involvement of both CB2Rs and non-CB2Rs in BCP’s action [38]. However, it is unknown whether BCP is effective in attenuation of cocaine taking and seeking. Therefore, in this study, we systemically evaluated the potential therapeutic utility of BCP for CUD using multiple animal models of drug addiction. In addition, we assessed the effects of BCP on nondrug (food) self-administration and possible BCP abuse liability using a drug substitution procedure.
Although a number of studies indicate that CB2R mechanisms may underlie the therapeutic effects of BCP in several disease models [30, 39, 40], more recent studies suggest that BCP has no binding affinity to CB1R and CB2Rs [41, 42], suggesting that non-CB2R mechanisms may also underlie BCP action. BCP was reported to have multiple non-CB2R targets, including µ-opioid receptors (MORs), toll-like receptor 4 (TLR-4), and peroxisome proliferated activator receptors (PPARα, PPARγ) [see a review by [29]]. Therefore, in this study, we used pharmacological and transgenic approaches to determine whether CB1Rs, CB2Rs, GPR55, or any of the above non-CB2Rs underlie BCP’s action in cocaine self-administration. Last, we used electrical and optical brain-stimulation reward paradigms to determine whether a dopamine-dependent mechanism is involved in BCP’s action. We found that BCP is effective in attenuation of cocaine taking and seeking by a dopamine-dependent mechanism related to stimulation of PPARα and PPARγ.
Materials and methods
Male Long–Evans rats, wild type C57BL/6 J mice, CB1-KO, CB2-KO, GPR55-KO, and DAT-cre mice were used in this study. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, National Academy of Sciences, and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse (see more details in S.I.)
Cocaine self-administration in rats and mice
Intravenous catheterization, viral surgeries, and cocaine self-administration procedures were performed, as described previously (7, 8). After stable cocaine self-administration was achieved, the effects of BCP on cocaine self-administration under an FR1 or FR2 schedule of reinforcement with multiple doses of cocaine in the presence or absence of a specific receptor antagonist were evaluated (see more details in the S.I.).
Electrical and optical brain-stimulation reward in rats and mice
Electrical stimulation of the medial forebrain bundle is rewarding in rats. Similarly, optogenetic stimulation of midbrain DA neurons is also rewarding in transgenic DAT-cre mice. Here we used both procedures to determine whether a DA-dependent mechanism underlying BCP’s action against cocaine (see more details in S.I.).
Drugs
Beta-caryophyllene was purchased from Sigma-Aldrich. All other receptor agonists and antagonists were purchased from Tocris (see more details in S.I.).
Results
BCP attenuates cocaine self-administration under an FR1 schedule of reinforcement in rats
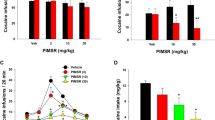
Figure 1a shows that systemic administration of BCP, at 100 mg/kg, but not at lower doses, reduced cocaine self-administration under an FR1 schedule of reinforcement. A one-way ANOVA revealed a significant BCP treatment main effect (F3,24 = 5.36; p = 0.05), which was driven by the 100 mg/kg BCP dose (p < 0.05).
a Intraperitoneal (i.p.) administration of BCP dose-dependently inhibited cocaine self-administration maintained by 0.5 mg/kg/infusion under an FR1 schedule of reinforcement (n = 8). b BCP inhibited cocaine self-administration under a FR2 schedule of reinforcement and shifted the cocaine dose-response curve downward (n = 8). c Intragastric (i.g.) administration of BCP (50, 100 mg/kg, i.g.) failed to inhibit cocaine self-administration maintained by 0.5 mg/kg/infusion (n = 6). d Intragastric administration of BCP dose-dependently inhibited cocaine self-administration maintained by lower doses of cocaine (n = 8). e BCP (i.p.) significantly reduced cocaine conditioned place preference (CPP) in rats (n = 8–10 per group). f BCP (i.p.) also reduced cocaine-primed (10 mg/kg) reinstatement of drug seeking in rats extinguished from cocaine self-administration (n = 8 per group). g BCP did not alter food pellet self-administration in rats (n = 8). h In a drug substitution test, when BCP substituted for cocaine, rats ceased responding (n = 8). *p < 0.05, compared to the vehicle control group (a, b, d, f) or pre-conditioning (e), or the phase of cocaine self-administration (h).
BCP downward shifts the cocaine dose-response curve in rats
To further determine a possible interaction of BCP and cocaine, we then assessed the effects of BCP on a cocaine dose-response curve in rats. Figure 1b shows a typical inverted U-shaped curve of cocaine dose-responses in the vehicle-treated group. Systemic (i.p.) treatment with BCP (25, 50, 100 mg/kg) dose-dependently decreased cocaine self-administration and shifted the cocaine dose-response curve downward. A two-way ANOVA with cocaine dose as a repeated-measures factor revealed a significant cocaine dose × BCP treatment interaction (F15,160 = 2.46, p < 0.05). Post-hoc Tukey tests for multiple group comparisons revealed that 50 and 100 mg/kg doses of BCP significantly reduced self-administration maintained by lower doses of cocaine (p < 0.05). (for more detailed statistical analysis, see Table 1 in S.I.)
Intragastric administration of BCP inhibits cocaine self-administration
To further explore the translational potential of BCP for the treatment of CUD in humans, it is important to determine whether intragastric administration of BCP is also effective in reducing cocaine self-administration. For this purpose, we first observed the effects of intragastric BCP on cocaine self-administration maintained by 0.5 mg/kg/infusion dose of cocaine. We found that BCP, within a dose range (50–200 mg/kg, i.g.), failed to inhibit cocaine self-administration maintained by a high dose of cocaine (0.5 mg/kg/infusion) (one-way ANOVA, p > 0.05) (Fig. 1c).
Next we lowered cocaine doses and found that intragastric administration of BCP (50–200 mg/kg) dose-dependently inhibited cocaine self-administration and shifted the cocaine dose-response curve downward (Fig. 1d, cocaine dose × BCP dose interaction, F15,140 = 3.18, p < 0.05). Post-hoc Tukey tests for multiple group comparisons indicated that BCP is effective in attenuation of cocaine self-administration maintained by lower doses of cocaine in a dose-dependent manner (Fig. 1d, p < 0.05) (for more detailed statistical analysis, see S.I.).
BCP reduces cocaine-induced CPP in rats
To confirm the above findings, we next used the CPP paradigm to determine whether BCP has similar anticocaine reward effect. Figure 1e shows the results from the CPP test, indicating that cocaine-paired conditioning produced significant place preference in the vehicle-treated rats, not in those treated with BCP (50 or 100 mg/kg) (two-way ANOVA, phase × BCP dose interaction: F2,24 = 3.49, p < 0.05) (for more detailed statistical analysis, see S.I.).
BCP reduces cocaine-induced reinstatement of drug seeking in rats
To determine whether BCP is also effective in preventing relapse to cocaine seeking, we used a drug-primed reinstatement paradigm that consisted of 3 phases: self-administration, extinction and a reinstatement test. Prior to the reinstatement test rats in 3 different groups were pretreated with 0, 25, or 50 mg/kg of BCP and 30 min later they received a systemic injection of cocaine (10 mg/kg, ip). Rats pretreated with vehicle showed robust reinstatement of cocaine seeking when primed with cocaine (Fig. 1f). However, those pretreated with 25 or 50 mg/kg dose of BCP did not show significant reinstatement responding on the active lever (Fig. 1f, one-way ANOVA: F2,17 = 5.76, p < 0.01), suggesting that BCP has therapeutic potential in relapse prevention.
BCP fails to affect cocaine-induced hyperlocomotion
We also examined the effects of BCP on cocaine-enhanced hyperactivity. We found that systemic administration of BCP (50, 100 mg/kg, i.p.) failed to alter cocaine (10 mg/kg)-induced hyperlocomotion in naive rats (Fig. S1). A two-way ANOVA (time × BCP dose) revealed a significant main effect of time (F17, 187 = 26.64, p < 0.001), but no time × dose interaction.
BCP has no significant effect on food self-administration in rats
We next examined whether BCP also inhibits nondrug (food pellet)-maintained lever responding under similar experimental conditions. Figure 1g shows that BCP, at 50 and 100 mg/kg, did not produce a significant reduction in food self-administration (one-way ANOVA: F2,35 = 2.52, p > 0.05).
BCP itself has low abuse liability
Next we examined whether BCP itself has rewarding or abuse potential, one of the most important obstacles in medication development for substance use disorders. In this experiment, we first trained rats to reliably self-administer cocaine as described above, and then replaced cocaine with BCP during daily self-administration sessions. As shown in Fig. 1h, rats readily self-administered cocaine maintained by 0.5 mg/kg/infusion dose. Substitution with BCP (at 0.5 or 1.0 mg/kg doses) failed to maintain lever pressing. When BCP was replaced back with cocaine after 6 days of the substitution tests, rats rapidly recovered their self-administration behavior. A two-way ANOVA on cocaine infusions revealed a significant phase × day interaction (F6,36 = 6.07; p < 0.01), which was driven by a reduction in drug infusions when BCP was substituted (at the 0.5 and 1 mg/kg dose). Similarly, a separate two-way ANOVA on active lever pressing revealed a significant phase × day interaction (F6,36 = 5.36; p < 0.01). No significant effects of BCP substitution across days were observed on inactive lever pressing (p > 0.05), suggesting that intravenous BCP did not cause sedative effects. These findings suggest that BCP has no abuse potential. For more detailed statistical analysis, see S.I.
BCP inhibits cocaine self-administration by non-cannabinoid receptor mechanisms
As stated above, earlier in vitro binding assays and behavioral studies suggest that CB2R mechanisms underlie the therapeutic potential of BCP in many disease models [29, 30]. However, this was challenged by recent findings indicating that non-CB1/non-CB2 receptor mechanisms might be critically involved in BCP’s action in vivo [30, 41]. To further address this issue, we used several cannabinoid receptor gene-knocked mice – CB1-KO, CB2-KO, and GPR55-KO [43] and examined whether these receptors might be involved in BCP action against cocaine self-administration. Figure 2 shows that genetic deletion of CB1, CB2, or GRP55 receptors failed to attenuate BCP-induced reduction in cocaine self-administration. In contrast, an enhanced reduction in cocaine self-administration was observed in CB1-KO and CB2-KO mice as compared to WT mice. Separate one-way ANOVAs with BCP dose as repeated-measures factor indicate that BCP induced a significant reduction in cocaine self-administration in WT mice (Fig. 2a, F2,48 = 4.76; p = 0.013), CB1-KO mice (Fig. 2b, F2,8 = 13.66; p < 0.01 ), CB2-KO (Fig. 2c, F2,25 = 29.56; p < 0.001), or GRP55-KO mice (Fig. 2d, F2,14 = 8.66; p = 0.004), suggesting that BCP-attenuating effects against cocaine are mediated by non-CB1, non-CB2, and non-GRP55 receptor mechanisms.
CB1, CB2, MOR, and TLR4 receptor antagonists failed to alter BCP action in cocaine self-administration
We then used pharmacological approaches to explore other potential receptor mechanisms involved in BCP efficacy in rats. Figure 3a shows that BCP (100 mg/kg) caused a significant downward shift of the cocaine dose–response curve, whereas pretreatment with AM251 (a CB1R antagonist, 3 mg/kg) failed to block BCP-attenuating effects in cocaine self-administration. A two-way ANOVA revealed a significant cocaine dose × treatment interaction (F10,115 = 3.76; p < 0.01). Post-hoc Tukey tests did not reveal significant differences in cocaine self-administration between (vehicle + BCP) and (AM251 + BCP) groups. Likewise, pretreatment with AM630 (a CB2R antagonist, 3 mg/kg) failed to block BCP-induced reductions in cocaine self-administration (Fig. 3b, two-way ANOVA, cocaine dose × treatment interaction: F10,110 = 3.00, p < 0.01). Post-hoc Tukey tests did not reveal significant differences in cocaine self-administration between (Veh + BCP) and (AM630 + BCP) groups. Figure 3c shows that pretreatment with naloxone (a MOR antagonist, 3 mg/kg) failed to alter BCP-induced reductions in cocaine self-administration (two-way ANOVA, treatment × cocaine dose interaction: F10,110 = 4.07, p < 0.001). Post-hoc Tukey tests did not reveal significant differences in cocaine self-administration between (Veh + BCP) and (Nalx + BCP) groups. Figure 3d shows pretreatment with the 3 mg/kg dose of TAK242 (a TLR-4 receptor antagonist) also failed to block BCP attenuating effects on cocaine self-administration (treatment × cocaine dose interaction: F10,115 = 2.84; p < 0.01). Post-hoc Tukey tests did not reveal significant differences in cocaine self-administration between (Veh + BCP) and (TAK242 + BCP) groups. For more detailed statistical analysis, see S.I.
Pretreatment with AM251 (a CB1 antagonist, 3 mg/kg) (a), AM630 (a CB2 antagonist, 3 mg/kg) (b), naloxone (NXL, a MOR antagonist, 3 mg/kg) (c), TAK242 (a TLR-4 antagonist, 3 mg/kg) (d) failed to block BCP-induced reduction in cocaine self-administration. In contrast, pretreatment with GW6471 (a PPARα antagonist, 3 mg/kg) (e) or GW9662 (a PPARγ antagonist, 3 mg/kg) (f) blocked BCP-induced reduction in cocaine self-administration. Pretreatment with GW7647 (a PPARα agonist, 3 and 5 mg/kg) (g) or pioglitazone (a PPARγ agonist, 10 mg/kg) (h) also reduced cocaine self-administration by itself. *p < 0.05, as compared to the vehicle control group at each cocaine dose.
PPARα and PPARγ antagonists block BCP’s action in cocaine self-administration
Unexpectedly, pretreatment with GW6471 (a PPARα antagonist, 3 mg/kg) blocked BCP-attenuating effects on cocaine self-administration (Fig. 3e). A two-way ANOVA revealed a significant cocaine dose × treatment interaction (F10,110 = 3.24; p < 0.01). Post-hoc Tukey tests for multiple group comparisons revealed that rats in the (Veh + BCP) group self-administered significantly less cocaine than the (Veh + Veh) control group and the (GW6471 + BCP) group (p < 0.05). There were no significant differences in the numbers of cocaine infusions between the (Veh + Veh) and (GW6471 + BCP) groups. Similarly, pretreatment with GW9662 (a PPARγ antagonist, 3 mg/kg) also prevented BCP (100 mg/kg)-induced reductions in cocaine self-administration (Fig. 3f; cocaine dose × treatment interaction: F10,100 = 2.15, p < 0.05). Post-hoc Tukey tests revealed that the (Veh + BCP) group self-administered significantly less cocaine than the (Veh + Veh) control or (GW9662 + BCP) groups (p < 0.05). These findings suggest that BCP attenuating effects on cocaine-self administration are involved in stimulation of PPARα and PPARγ receptors. For more detailed statistical analysis, see S.I.
To confirm these findings, we tested whether systemic administration of PPARα or PPARγ agonist inhibits cocaine self-administration in a way similar to BCP. Figure 3g shows that pretreatment with GW7647 (a selective PPARα agonist, 3 or 5 mg/kg) significantly reduced cocaine self-administration and shifted the cocaine dose-response curve downward in a dose-dependent manner. A two-way ANOVA indicated a significant main effect of GW7647 treatment (F10,110 = 2.35, p < 0.05). Similarly, pretreatment with pioglitazone (10 mg/kg) also caused a significant reduction in cocaine self-administration (Fig. 3h, F5,75 = 2.68; p < 0.05). These findings suggest that stimulation of PPARα or PPARγ causes a reduction in cocaine self-administration, which provide additional evidence supporting the involvement of PPARα and PPARγ mechanisms in BCP’s anticocaine effects. For more detailed statistical analysis, see S.I.
BCP failed to reduce cocaine-enhanced electrical brain-stimulation reward in rats
How do PPARα and PPARγ underlie BCP’s action in cocaine self-administration? It was recently reported that PPARα and PPARγ may modulate the mesolimbic DA system [44, 45]. Thus, we hypothesized that BCP action against cocaine may involve the mesolimbic DA system. To test this hypothesis, we used an electrical brain-stimulation reward (BSR) paradigm to observe a possible interaction between cocaine and BCP. We found that electrical stimulation of the medial forebrain bundle (MFB) (Fig. 4a) caused robust BSR. Figure 4b shows typical rate-frequency functions for BSR, indicating the BSR threshold θ0 and Ymax. Cocaine (2 mg/kg, i.p.) shifted the stimulation-response curve to the left and decreased the BSR threshold θ0 value, but not affecting rates of responding (Ymax) (Fig. 4c). A one-way ANOVA revealed a significant cocaine treatment main effect (Fig. 4c, F3,21 = 5.81, p < 0.01, compared to vehicle). However, BCP pretreatment did not produce a statistically significant reduction in the θ0 value (Fig. 4c, F2,14 = 0.755, p > 0.05, compared to vehicle) nor in the Ymax value (Fig. 4d, F2,14 = 0.43, p > 0.05).
a A diagram showing that electrical stimulation of the medial forebrain bundle at the hypothalamus causes intracranial self-stimulation (ICSS) behavior (known as BSR). b The representative stimulation–response curves, indicating that cocaine shifted the stimulation-response curve to the left and decreased the BSR stimulation threshold (θ0 value), while BCP shifted the stimulation–response curve rightward. c Averaged % changes in the stimulation threshold (θ0 value), indicating that cocaine significantly decreased the θ0 value (p < 0.05), while pretreatment with BCP failed to reverse cocaine-induced reduction in θ0 value (p > 0.05). d BCP pretreatment did not alter Ymax in the presence or absence of cocaine. *p < 0.005, **p < 0.01, as compared to the vehicle treatment group.
BCP reduces DA-dependent optical brain-stimulation reward in DAT-cre mice
To determine whether the failure of BCP to inhibit cocaine-enhanced BSR was caused by electrical activation of non-dopaminergic fibers in the MFB, we used an optogenetic ICSS paradigm to selectively activate VTA DA neurons in DAT-cre mice. Figure 5a shows the general experimental set-up, illustrating that AAV-ChR2 was microinjected into the VTA (bilateral) and then fibers were surgically implanted 0.5 mm above the VTA in DAT-cre mice. Figure 5b shows the fluorescent ChR2 expression in VTA DA neurons. Systemic administration of cocaine shifted the optical stimulation-response curve to the left, which was dose-dependently blocked by pretreatment with BCP (Fig. 5c, two-way ANOVA: treatment × frequency interaction: F15,75 = 4.33, p < 0.001). Post-hoc tests for multiple group comparisons indicated that BCP pretreatment significantly attenuated cocaine’s action in oICSS (p < 0.05). BCP alone (50, 100 mg/kg, i.p.) caused a significant reduction in optical ICSS (Fig. 5d, two-way ANOVA: BCP dose × frequency interaction: F10,50 = 9.14, p < 0.001). Post-hoc Tukey tests revealed significant reductions in oICSS maintained by 50 and 100 Hz stimulation (p < 0.05). For more detailed statistical analysis, see S.I.
a A diagram showing the experimental methods for oICSS. AAV-ChR2-eYFP viruses were microinjected into the VTA of DAT-cre mice and fibers were implanted into the VTA to optically excite VTA DA neurons contingently upon lever response. b Representative images of TH-immunostaining (red) and fluorescent ChR2-EYFP expression (green) in the VTA, illustrating ChR2-EYFP expression in VTA DA neurons. c Representative event oICSS records of lever-pressing for descending stimulation frequencies in a representative session (from 100 Hz to 1 Hz, 10 min per frequency), indicating that photoactivation of VTA dopamine neurons induces robust oICSS behavior in a stimulation frequency-dependent manner. d The averaged stimulation-response curves, indicating that cocaine shifted the curve upward, which was then blocked by BCP in a dose-dependent manner. e BCP alone dose-dependently shifted the stimulation-response curve downward, indicating a reduction in BSR. *p < 0.05, as compared to the baseline (d) or the vehicle control group (e).
Discussion
In a series of experiments, we evaluated the potential utility of BCP as a repurposed drug for the treatment of CUD and explored the underlying receptor mechanisms of actions. We found that: (1) BCP significantly reduced cocaine self-administration in rats and mice when administered intraperitoneally or orally (intragastrically). In addition, BCP inhibited cocaine CPP and cocaine-primed reinstatement of drug seeking, but not cocaine-enhanced locomotion or food self-administration. BCP substitution for cocaine failed to maintain self-administration, suggesting that BCP has anticocaine therapeutic potential without exerting abuse potential; (2) Genetic deletion of CB1, CB2, or GPR55 receptors in respective CB1-KO, CB2-KO or GPR55-KO mice failed to block BCP-induced reductions in cocaine self-administration. Pharmacological blockade of CB1, CB2, MOR, or TLR-4 receptors also failed to block BCP’s anticocaine effects, suggesting that these receptors are not involved in BCP’s action; (3) In contrast, blockade of PPARα or PPARγ attenuated BCP-induced reductions in cocaine self-administration, while selective stimulation of PPARα or PPARγ inhibited cocaine self-administration in a way similar to BCP. These data suggest that BCP’s therapeutic effects are mediated at least partially by stimulation of PPARα or PPARγ; (4) Last, although BCP did not significantly affect cocaine-enhanced electrical BSR in rats, but dose-dependently reduced cocaine-enhanced BSR maintained by optical stimulation of VTA DA neurons in DAT-cre mice, suggesting that a DA mechanism is involved in BCP’s action. Together, these findings suggest that BCP has promising therapeutic potential for cocaine abuse and addiction.
BCP: An emerging frontier in medication development for CNS disorders
BCP did not gain attention of the scientific community until the 2008 report that BCP acts as a selective CB2R agonist and produces anti-inflammatory effects via a CB2R mechanism [30]. BCP is a widely recognized phytocannabinoid or terpene, found in a variety of spice and food plants [29]. Due to its distinctive flavor, fragrance, and an excellent safety profile, BCP has been approved by the FDA as a “generally recognized as safe” food or cosmetic additive. BCP offers significant therapeutic benefits for a wide range of medical conditions including inflammation [30, 32], neuropathic pain [33, 46], diabetes [47, 48], anxiety [34], depression [34, 49], multiple sclerosis [50, 51], cancer [52, 53], Alzheimer’s disease [54, 55], Parkinson’s disease [56,57,58] and bacterial infections [59, 60]. Although a majority of these findings come from preclinical work, there is also clinical evidence that pure BCP or BCP rich essential oils reduces hand arthritis [61], nausea and epigastric pain [62] and menstrual cramps [63].
In addition, the literature suggests that BCP might have positive impact on addiction-like behaviors. For example, BCP has been shown to reduce alcohol consumption and alcohol CPP [64] as well as nicotine taking and seeking in rodents [38]. Here we report that BCP produced attenuating effects on cocaine self-administration and reinstatement of cocaine seeking. Notably, BCP inhibited cocaine self-administration when administered systemically or intragastrically, making it an attractive candidate in translational research [65]. These results are especially encouraging as BCP is a safe phytocannabinoid with a low toxicity profile [66, 67] and good oral bioavailability [40]. In addition, BCP has moderate selectivity (~60-fold) for CB2 over CB1Rs [30] without psychotomimetic effects [66]. As demonstrated in this study, BCP itself has no abuse potential. Given its multifaceted medical benefits [29, 37], unique pharmacological profile and being approved by the FDA, BCP is emerging as a frontier in medication development programs.
We note some discrepancies regarding the BCP efficacy observed in different experiments. For example, BCP dose-dependently inhibited self-administration maintained by 0.5 mg/kg/infusion dose of cocaine in a classical single dose cocaine self-administration paradigm (Fig. 1a), but not in a multiple-cocaine dose self-administration paradigm (Fig. 1-b, d). This may be related to different procedures used. In the latter paradigm, higher doses of cocaine (0.25, 0.5 mg/kg/infusion) were presented last (i.e., 2 h after the BCP treatment). Thus, it is likely that BCP effects might have worn off at this point. This is supported by our previous finding that BCP inhibits nicotine self-administration and this effect lasted for about 60–80 min [38]. We also note that BCP produced a descending trend in food self-administration in rats, which is somehow different from our previous report indicating that BCP significantly inhibited food-self-administration in mice [38]. BCP also failed to alter an acute high dose of cocaine-enhanced locomotion in drug naïve rats. Such discrepancy might be related to differences in species (rats vs. mice), drug (BCP, cocaine) doses, long-time cocaine self-administration-induced neuroadaptations, and methodology (time durations, maximally allowed deliveries, food deprivation or not) used in these two studies.
Non-CB2 receptor mechanisms underlying BCP action in cocaine self-administration
BCP has been initially identified as a moderately potent (Ki = 155 nM) and selective CB2R agonist with 66-fold selectivity for CB2R over CB1R [30] and its analgesic [32, 68], anxiolytic, anti-depressant [34], and anti-inflammatory effects [56] have been attributed to stimulation of CB2Rs [30, 32, 50, 68]. In addition, we and others have previously reported that JWH133, a highly potent (Ki = 1.3 nM) and selective CB2R agonist (~200-fold selectivity for CB2R over CB1R) [69], significantly reduces cocaine self-administration in rats and mice [8, 12, 70], cocaine-induced CPP [9, 10], sensitization [10] and hyperlocomotion [71]. Furthermore, functional CB2 receptors are identified on cell bodies of VTA dopamine neurons [12, 72] and their terminals in the NAc [25, 73]. Therefore, we initially predicted that a CB2R mechanism might underlie BCP’s action in cocaine self-administration.
Surprisingly, we found that genetic deletion or pharmacological blockade of CB1 or CB2 receptor failed to block BCP’s action against cocaine self-administration, which directly challenges our initial hypothesis. The reasons for such conflicting findings are unclear. One possibility is that BCP is not a potent CB2R agonist as JWH133. Therefore, stimulation of CB2Rs alone by BCP is not sufficient in attenuating cocaine self-administration. We also note recent reports that BCP has no binding affinity to either CB1Rs or CB2Rs [41, 42]. These might explain why pharmacological blockade or genetic deletion of CB2Rs failed to alter BCP action against cocaine self-administration in this study. We have recently reported that the underlying receptor mechanisms of BCP action depends on drugs of abuse and BCP doses – lower doses of BCP (25 mg/kg, i.p.) inhibited nicotine self-administration via CB2Rs, while high doses of BCP produced a more potent dose-dependent reduction in nicotine self-administration by both CB2 and non-CB2 receptor mechnisms (33). These conclusions were drawn based on our previous findings that genetic deletion or pharmacological blockade of CB2R prevented the BCP effects at low (25 mg/kg), but not high doses (50, 100 mg/kg), against nicotine self-administration [38]. Low doses of BCP can inhibit nicotine, but not cocaine, self-administration. This may be related to a fact that cocaine is more reinforcing than nicotine. Thus, higher doses of BCP might be required to block cocaine action. This is supported by our findings. However, higher effective doses of BCP also act on non-CB2 receptors.
Since BCP has been initially recognized as a cannabinoid [30] and now as a terpenoid [38, 42], we also examined the possible involvement of other putative cannabinoids such as CB1Rs and GPR55 (a putative cannabinoid receptor) in BCP’s action. We found that genetic deletion or pharmacological blockade of CB1 or GPR55 receptors failed to alter BCP’s action in cocaine self-administration.
PPARα and PPARγ mechanisms underlie BCP’s action against cocaine
In addition, recent reports indicate that therapeutic effects of BCP in various disease models might be mediated by action on MOR, TLR-4, and PPARα/γ receptors [29, 33, 35, 74,75,76,77]. Therefore, we examined the role of these receptors in BCP’s action against cocaine self-administration. We found that pharmacological blockade of either MORs or TLR-4 failed to alter BCP-induced reduction in cocaine self-administration. Unexpectedly, blockade of PPARα or PPARγ with selective antagonists blocked BCP-attenuating effects on cocaine self-administration, suggesting the involvement of these two receptors in BCP’s action. Likewise, under the same experimental conditions, pretreatment with a PPARα or PPARγ agonist also inhibited cocaine self-administration. These findings are consistent with previous reports that PPARα agonists can reduce alcohol consumption [78], nicotine self-administration [79,80,81] and cocaine-related effects. Likewise, PPARγ agonists were reported to reduce heroin self-administration [44], heroin- [82] and cocaine-seeking in rats and these effects could be blocked by the PPARγ antagonist GW9662 [83]. In addition, PPARγ has been implicated in craving for cocaine in addicted individuals [84]. Activation of PPARγ can prevent physical and affective signs of withdrawal from nicotine [85], opioids [82], or prevent the development of behavioral sensitization to methamphetamine [86]. These findings suggest that PPARα and/or PPARγ could be important targets of BCP against cocaine or other drugs of abuse. We note that some attempts to translate PPAR ligands (e.g., gemfibrozil or pioglitazone) into a clinical setting for the treatment of alcoholism and nicotine addiction have not been successful [87]. Therefore, more studies are needed to determine whether BCP, an FDA-approved terpene with similar PPARα and PPARγ agonist-like profiles, has translational potential for the treatment of substance use disorders.
DA-dependent mechanisms underlying BCP’s action in cocaine self-administration
It is unknown how PPARα and PPARγ are involved in BCP’s action. N-acylethanolamines, such as oleoylethanolamide and palmitoylethanolamide, may act as endogenous ligands of PPARα [88, 89] and anandamide may act as an endogenous ligand of PPARγ [90]. Thus, one possibility is that BCP might alter such endocannabinoid release, which subsequently stimulates PPARα and PPARγ, producing an inhibitory effect on drug reward and relapse. Immunocytochemistry assays indicate high levels of PPARα expression in the prefrontal cortex, striatum, VTA [91, 92] and high PPARγ expression in the VTA [44, 93]. Electrophysiological evidence suggests that PPARα regulates VTA DA neuronal activity through nicotinic receptors [89, 94]. Within the VTA, PPARγ are identified in the areas near to GABAergic neurons and VTA GABA terminals and in the rostromedial tegmental nucleus, suggesting that PPARγ may initially modulate (increase) GABA release that subsequently modulates (decreases) the mesolimbic DA release in the NAc [94, 95].
To test this DA hypothesis, we first used electrical ICSS paradigm to observe the interaction of cocaine and BCP on BSR in rats. We found that cocaine enhanced electrical BSR but BCP pretreatment failed to produce a significant decrease against cocaine’s action. Alternatively, we used an optical ICSS paradigm in DAT-cre mice, in which light-sensitive ChR2 proteins are expressed in VTA DA neurons. This allows us to selectively stimulate VTA DA neurons and observe DA-dependent ICSS behavior. In this study, we found that BCP inhibited optical ICSS and dose-dependently attenuated cocaine-enhanced BSR, suggesting a DA-dependent mechanism that might be involved in BCP’s action. The different findings in electrical versus optical ICSS experiments may be related to other non-DA mechanisms caused by nonspecific electrical stimulation of the medial forebrain bundle. We should point out that BCP action is not cocaine specific. As mentioned above, BCP also inhibits nicotine self-administration [38], alcohol CPP and intake [64], and oICSS as shown in the present study. It is conceivable that BCP produces its therapeutic effects by stimulating PPARα and/or PPARγ [96, 97] within the mesolimbic reward (DA) system, a core system critically involved in the rewarding effects of drugs of abuse and oICSS.
In conclusion, the present findings suggest that BCP has therapeutic potential for the treatment of CUD, as assessed in multiple animal models of cocaine addiction. In addition, BCP does not produce adverse effects such as sedation nor exerts abusive liability. Importantly, BCP is effective in inhibition of cocaine self-administration after systemic or intragastrical administration. Furthermore, a series of mechanistic studies suggest that PPARα and PPARγ, rather than CB2R or other receptors, might underlie the anticocaine effects of BCP. Given that BCP is an FDA-approved dietary additive with a good safety profile, we believe that BCP deserves further study as a promising repurposing drug for the treatment of CUD.
Funding and disclosure
This research was supported by the National Institute on Drug Abuse Intramural Research Program (Z1A DA000633-01). None of the authors have any conflicts of interest.
References
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73.
Galaj E, Xi Z-X. Potential of cannabinoid receptor ligands as treatment for substance use disorders. CNS Drugs. 2019;33:1001–30.
Manzanares J, Cabañero D, Puente N, García-Gutiérrez MS, Grandes P, Maldonado R. Role of the endocannabinoid system in drug addiction. Biochem Pharmacol. 2018;157:108–21.
Arnold JC. The role of endocannabinoid transmission in cocaine addiction. Pharm Biochem Behav. 2005;81:396–406.
Galaj E, Bi G-H, Yang H-J, Xi Z-X. Cannabidiol attenuates the rewarding effects of cocaine in rats by CB2, 5-HT1A and TRPV1 receptor mechanisms. Neuropharmacology. 2020;167:107740.
Wiskerke J, Pattij T, Schoffelmeer ANM, De, Vries TJ. The role of CB1 receptors in psychostimulant addiction. Addict Biol. 2008;13:225–38.
Xi Z-X, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, et al. Cannabinoid cb1 receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology. 2008;33:1735–45.
Xi Z-X, Peng X-Q, Li X, Song R, Zhang H-Y, Liu Q-R, et al. Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–6.
Delis F, Polissidis A, Poulia N, Justinova Z, Nomikos GG, Goldberg SR, et al. Attenuation of cocaine-induced conditioned place preference and motor activity via cannabinoid cb2 receptor agonism and cb1 receptor antagonism in rats. Int J Neuropsychopharmacol. 2017;20:269–78.
Lopes JB, Bastos JR, Costa RB, Aguiar DC, Moreira FA. The roles of cannabinoid CB1 and CB2 receptors in cocaine-induced behavioral sensitization and conditioned place preference in mice. Psychopharmacol. (Berl) 2020;237:385–94.
Yu L-L, Zhou S-J, Wang X-Y, Liu J-F, Xue Y-X, Jiang W, et al. Effects of cannabinoid CB1 receptor antagonist rimonabant on acquisition and reinstatement of psychostimulant reward memory in mice. Behav Brain. Res 2011;217:111–6.
Zhang H-Y, Gao M, Liu Q-R, Bi G-H, Li X, Yang H-J, et al. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci USA. 2014;111:E5007–5015.
De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–4.
Ward SJ, Rosenberg M, Dykstra LA, Walker EA. The CB1 antagonist rimonabant (SR141716) blocks cue-induced reinstatement of cocaine seeking and other context and extinction phenomena predictive of relapse. Drug Alcohol Depend. 2009;105:248–55.
Xi Z-X, Gilbert JG, Peng X-Q, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–6.
Lesscher HMB, Hoogveld E, Burbach JPH, van Ree JM, Gerrits MAFM. Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol. 2005;15:31–37.
Cossu G, Ledent C, Fattore L, Imperato A, Böhme GA, Parmentier M, et al. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65.
Fattore L, Martellotta MC, Cossu G, Mascia MS, Fratta W. CB1 cannabinoid receptor agonist WIN 55,212-2 decreases intravenous cocaine self-administration in rats. Behav Brain Res. 1999;104:141–6.
Caillé S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–702.
Filip M, Gołda A, Zaniewska M, McCreary AC, Nowak E, Kolasiewicz W, et al. Involvement of cannabinoid CB1 receptors in drug addiction: effects of rimonabant on behavioral responses induced by cocaine. Pharm Rep. 2006;58:806–19.
Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–4.
Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12:4038–46.
Miller LL, Ward SJ, Dykstra LA. Chronic unpredictable stress enhances cocaine-conditioned place preference in type 1 cannabinoid receptor knockout mice. Behav Pharmacol. 2008;19:575–81.
Li X, Hoffman AF, Peng X-Q, Lupica CR, Gardner EL, Xi Z-X. Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice. Psychopharmacol (Berl). 2009;204:1–11.
Aracil-Fernández A, Trigo JM, García-Gutiérrez MS, Ortega-Álvaro A, Ternianov A, Navarro D, et al. Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB2 receptors. Neuropsychopharmacology. 2012;37:1749–63.
Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology. 2009;205:171–4.
Jordan CJ, Xi Z-X. Progress in brain cannabinoid CB2 receptor research: From genes to behavior. Neurosci Biobehav Rev. 2019;98:208–20.
Mediavilla V, Steinemann S. Essential oil of cannabis sativa L. strains. I Int Hemp Res. 1997;4:80–2.
Sharma C, Al Kaabi JM, Nurulain SM, Goyal SN, Kamal MA, Ojha S. Polypharmacological properties and therapeutic potential of β-caryophyllene: a dietary phytocannabinoid of pharmaceutical promise. Curr Pharm Des. 2016;22:3237–64.
Gertsch J, Leonti M, Raduner S, Racz I, Chen J-Z, Xie X-Q, et al. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci USA. 2008;105:9099–104.
Cho JY, Chang H-J, Lee S-K, Kim H-J, Hwang J-K, Chun HS. Amelioration of dextran sulfate sodium-induced colitis in mice by oral administration of beta-caryophyllene, a sesquiterpene. Life Sci. 2007;80:932–9.
Klauke A-L, Racz I, Pradier B, Markert A, Zimmer AM, Gertsch J, et al. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur Neuropsychopharmacol. 2014;24:608–20.
Katsuyama S, Mizoguchi H, Kuwahata H, Komatsu T, Nagaoka K, Nakamura H, et al. Involvement of peripheral cannabinoid and opioid receptors in β-caryophyllene-induced antinociception. Eur J Pain. 2013;17:664–75.
Bahi A, Al Mansouri S, Al Memari E, Al Ameri M, Nurulain SM, Ojha S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol Behav. 2014;135:119–24.
Cheng Y, Dong Z, Liu S. β-caryophyllene ameliorates the alzheimer-like phenotype in APP/PS1 mice through CB2 receptor activation and the PPARγ pathway. Pharmacology. 2014;94:1–12.
Guo K, Mou X, Huang J, Xiong N, Li H. Trans-caryophyllene suppresses hypoxia-induced neuroinflammatory responses by inhibiting NF-κB activation in microglia. J Mol Neurosci. 2014;54:41–8.
Fidyt K, Fiedorowicz A, Strządała L, Szumny A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016;5:3007–17.
He Y, Galaj E, Bi G-H, Wang X-F, Gardner E, Xi Z-X. β-caryophyllene, a dietary terpenoid, inhibits nicotine taking and nicotine seeking in rodents. Br J Pharmacol. 2020;177:2058–72.
Bento AF, Marcon R, Dutra RC, Claudino RF, Cola M, Pereira Leite DF, et al. β-Caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARγ pathway. Am J Pathol. 2011;178:1153–66.
Varga ZV, Matyas C, Erdelyi K, Cinar R, Nieri D, Chicca A, et al. β‐Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. Br J Pharmacol. 2018;175:320–34.
Finlay DB, Sircombe KJ, Nimick M, Jones C, Glass M. Terpenoids From cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Front Pharmacol. 2020;11:359. https://doi.org/10.3389/fphar.2020.00359.
Santiago M, Sachdev S, Arnold JC, McGregor IS, Connor M. Absence of entourage: terpenoids commonly found in cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors. Cannabis Cannabinoid Res. 2019;4:165–76.
Wang X-F, Galaj E, Bi G-H, Zhang C, He Y, Zhan J, et al. Different receptor mechanisms underlying phytocannabinoid- versus synthetic cannabinoid-induced tetrad effects: opposite roles of CB1 /CB2 versus GPR55 receptors. Br J Pharmacol. 2020;177:1865–80.
de Guglielmo G, Melis M, De Luca MA, Kallupi M, Li HW, Niswender K, et al. PPARγ activation attenuates opioid consumption and modulates mesolimbic dopamine transmission. Neuropsychopharmacology. 2015;40:927–37.
Scheggi S, Melis M, De Felice M, Aroni S, Muntoni AL, Pelliccia T, et al. PPARα modulation of mesolimbic dopamine transmission rescues depression-related behaviors. Neuropharmacology. 2016;110:251–9.
Paula-Freire LIG, Andersen ML, Gama VS, Molska GR, Carlini ELA. The oral administration of trans-caryophyllene attenuates acute and chronic pain in mice. Phytomedicine. 2014;21:356–62.
Basha RH, Sankaranarayanan C. β-Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem Biol Interact. 2016;245:50–8.
Suijun W, Zhen Y, Ying G, Yanfang W. A role for trans-caryophyllene in the moderation of insulin secretion. Biochem Biophys Res Commun. 2014;444:451–4.
Hwang E-S, Kim H-B, Lee S, Kim M-J, Kim K-J, Han G, et al. Antidepressant-like effects of β-caryophyllene on restraint plus stress-induced depression. Behav Brain Res. 2020;380:112439.
Alberti TB, Barbosa WLR, Vieira JLF, Raposo NRB, Dutra RC. (-)-β-caryophyllene, a cb2 receptor-selective phytocannabinoid, suppresses motor paralysis and neuroinflammation in a murine model of multiple sclerosis. Int J Mol Sci. 2017;18:691. https://doi.org/10.3390/ijms18040691.
Fontes LBA, Dias DDS, Aarestrup BJV, Aarestrup FM, Da Silva Filho AA, Corrêa JO, et al. β-Caryophyllene ameliorates the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. Biomed Pharmacother. 2017;91:257–64.
Dahham SS, Tabana YM, Iqbal MA, Ahamed MBK, Ezzat MO, Majid ASA, et al. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of aquilaria crassna. Molecules. 2015;20:11808–29.
Legault J, Pichette A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol. 2007;59:1643–7.
Canseco-Alba A, Schanz N, Sanabria B, Zhao J, Lin Z, Liu Q-R, et al. Behavioral effects of psychostimulants in mutant mice with cell-type specific deletion of CB2 cannabinoid receptors in dopamine neurons. Behav Brain Res. 2018;360:286–97.
Hu Y, Zeng Z, Wang B, Guo S. Trans-caryophyllene inhibits amyloid β (Aβ) oligomer-induced neuroinflammation in BV-2 microglial cells. Int Immunopharmacol. 2017;51:91–8.
Javed H, Azimullah S, Haque ME, Ojha SK. Cannabinoid Type 2 (CB2) Receptors activation protects against oxidative stress and neuroinflammation associated dopaminergic neurodegeneration in rotenone model of parkinson’s disease. Front Neurosci. 2016;10:321.
Ojha S, Javed H, Azimullah S, Haque ME. β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol Cell Biochem. 2016;418:59–70.
Viveros-Paredes JM, González-Castañeda RE, Gertsch J, Chaparro-Huerta V, López-Roa RI, Vázquez-Valls E, et al. Neuroprotective Effects of β-caryophyllene against dopaminergic neuron injury in a murine model of Parkinson’s disease induced by MPTP. Pharmaceutical. 2017;10:60. https://doi.org/10.3390/ph10030060.
Kim YS, Park SJ, Lee EJ, Cerbo RM, Lee SM, Ryu CH, et al. Antibacterial compounds from Rose Bengal-sensitized photooxidation of beta-caryophyllene. J Food Sci. 2008;73:C540–5.
Pieri FA, Souza MC de C, Vermelho LLR, Vermelho MLR, Perciano PG, et al. Use of β-caryophyllene to combat bacterial dental plaque formation in dogs. BMC Vet Res. 2016;12:216. https://doi.org/10.1186/s12917-016-0842-1.
Bahr T, Allred K, Martinez D, Rodriguez D, Winterton P. Effects of a massage-like essential oil application procedure using copaiba and deep blue oils in individuals with hand arthritis. Complement Ther Clin Pract. 2018;33:170–6.
Shim HI, Song DJ, Shin CM, Yoon H, Park YS, Kim N. et al. [Inhibitory effects of β-caryophyllene on helicobacter pylori infection: a randomized double-blind, placebo-controlled study]. Korean J Gastroenterol.2019;74:199–204.
Ou M-C, Hsu T-F, Lai AC, Lin Y-T, Lin C-C. Pain relief assessment by aromatic essential oil massage on outpatients with primary dysmenorrhea: a randomized, double-blind clinical trial. J Obstet Gynaecol Res. 2012;38:817–22.
Al Mansouri S, Ojha S, Al Maamari E, Al Ameri M, Nurulain SM, Bahi A. The cannabinoid receptor 2 agonist, β-caryophyllene, reduced voluntary alcohol intake and attenuated ethanol-induced place preference and sensitivity in mice. Pharm Biochem Behav. 2014;124:260–8.
Santos PS, Oliveira TC, R Júnior LM, Figueiras A, Nunes LCC. β-caryophyllene delivery systems: enhancing the oral pharmacokinetic and stability. Curr Pharm Des. 2018;24:3440–53.
Schmitt D, Levy R, Carroll B. Toxicological evaluation of β-caryophyllene oil: subchronic toxicity in rats. Int J Toxicol 2016;35:558–67.
Oliveira GL, da S, Machado KC, Machado KC, da Silva APDSCL, Feitosa CM, et al. Non-clinical toxicity of β-caryophyllene, a dietary cannabinoid: absence of adverse effects in female Swiss mice. Regul Toxicol Pharmacol 2018;92:338–46.
Aly E, Khajah MA, Masocha W. β-Caryophyllene, a CB2-receptor-selective phytocannabinoid, suppresses mechanical allodynia in a mouse model of antiretroviral-induced neuropathic pain. Molecules. 2019;25:106. https://doi.org/10.3390/molecules25010106.
Huffman JW. CB2 receptor ligands. Mini Rev Med Chem. 2005;5:641–9.
Zhang H-Y, Bi G-H, Li X, Li J, Qu H, Zhang S-J, et al. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology. 2015;40:1037–51.
Gobira PH, Oliveira AC, Gomes JS, da Silveira VT, Asth L, Bastos JR, et al. Opposing roles of CB1 and CB2 cannabinoid receptors in the stimulant and rewarding effects of cocaine. Br J Pharmacol. 2018. 12, 10.1111/bph.14473.
Zhang H-Y, Gao M, Shen H, Bi G-H, Yang H-J, Liu Q-R, et al. Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addict Biol. 2017;22:752–65.
Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, et al. antipsychotic-like effects of m4 positive allosteric modulators are mediated by CB2 receptor-dependent inhibition of dopamine release. Neuron. 2016;91:1244–52.
Cho H-I, Hong J-M, Choi J-W, Choi H-S, Kwak JH, Lee D-U, et al. β-caryophyllene alleviates D-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the TLR4 and RAGE signaling pathways. Eur J Pharmacol. 2015;764:613–21.
Irrera N, D’Ascola A, Pallio G, Bitto A, Mazzon E, Mannino F, et al. β-caryophyllene mitigates collagen antibody induced arthritis (CAIA) in mice through a cross-talk between CB2 and PPAR-γ receptors. Life Sci. 2019;237:116915. https://doi.org/10.1016/j.lfs.2019.116915.
Tian X, Liu H, Xiang F, Xu L, Dong Z. β-Caryophyllene protects against ischemic stroke by promoting polarization of microglia toward M2 phenotype via the TLR4 pathway. Life Sci. 2019;237:116915.
Wu C, Jia Y, Lee JH, Jun H, Lee H-S, Hwang K-Y, et al. Trans-caryophyllene is a natural agonistic ligand for peroxisome proliferator-activated receptor-α. Bioorg Med Chem Lett. 2014;24:3168–74.
Haile CN, Kosten TA. The peroxisome proliferator-activated receptor alpha agonist fenofibrate attenuates alcohol self-administration in rats. Neuropharmacology. 2017;116:364–70.
Jackson A, Bagdas D, Muldoon PP, Lichtman AH, Carroll FI, Greenwald M, et al. In vivo interactions between α7 nicotinic acetylcholine receptor and nuclear peroxisome proliferator-activated receptor-α: Implication for nicotine dependence. Neuropharmacology. 2017;118:38–45.
Le Foll B, Di Ciano P, Panlilio LV, Goldberg SR, Ciccocioppo R. Peroxisome proliferator-activated receptor (PPAR) agonists as promising new medications for drug addiction: preclinical evidence. Curr Drug Targets. 2013;14:768–76.
Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, et al. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry. 2011;69:633–41.
de Guglielmo G, Kallupi M, Scuppa G, Demopulos G, Gaitanaris G, Ciccocioppo R. Pioglitazone attenuates the opioid withdrawal and vulnerability to relapse to heroin seeking in rodents. Psychopharmacol. 2017;234:223–34.
Miller WR, Fox RG, Stutz SJ, Lane SD, Denner L, Cunningham KA, et al. PPARγ agonism attenuates cocaine cue reactivity. Addict Biol. 2018;23:55–68.
Schmitz JM, Green CE, Hasan KM, Vincent J, Suchting R, Weaver MF, et al. PPAR-gamma agonist pioglitazone modifies craving intensity and brain white matter integrity in patients with primary cocaine use disorder: a double-blind randomized controlled pilot trial. Addiction. 2017;112:1861–8.
Domi E, Caputi FF, Romualdi P, Domi A, Scuppa G, Candeletti S, et al. Activation of PPARγ attenuates the expression of physical and affective nicotine withdrawal symptoms through mechanisms involving amygdala and hippocampus neurotransmission. J Neurosci. 2019;39:9864–75.
Maeda T, Kiguchi N, Fukazawa Y, Yamamoto A, Ozaki M, Kishioka S. Peroxisome proliferator-activated receptor gamma activation relieves expression of behavioral sensitization to methamphetamine in mice. Neuropsychopharmacology. 2007;32:1133–40.
Gendy MNS, Di Ciano P, Kowalczyk WJ, Barrett SP, George TP, Heishman S, et al. Testing the PPAR hypothesis of tobacco use disorder in humans: a randomized trial of the impact of gemfibrozil (a partial PPARα agonist) in smokers. PLoS ONE. 2018;13:e0201512.
Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodríguez De Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–3.
Melis M, Carta G, Pistis M, Banni S. Physiological role of peroxisome proliferator-activated receptors type α on dopamine systems. CNS Neurol Disord Drug Targets. 2013;12:70–7.
Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–81.
Moreno S, Farioli-Vecchioli S, Cerù MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–45.
Warden A, Truitt J, Merriman M, Ponomareva O, Jameson K, Ferguson LB, et al. Localization of PPAR isotypes in the adult mouse and human brain. Sci Rep. 2016;6:27618.
Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD, et al. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology. 2009;150:707–12.
Melis M, Carta S, Fattore L, Tolu S, Yasar S, Goldberg SR, et al. Peroxisome proliferator-activated receptors-alpha modulate dopamine cell activity through nicotinic receptors. Biol Psychiatry. 2010;68:256–64.
Luchicchi A, Lecca S, Carta S, Pillolla G, Muntoni AL, Yasar S, et al. Effects of fatty acid amide hydrolase inhibition on neuronal responses to nicotine, cocaine and morphine in the nucleus accumbens shell and ventral tegmental area: involvement of PPAR-alpha nuclear receptors. Addict Biol. 2010;15:277–88.
Wenzel JM, Cheer JF. Endocannabinoid regulation of reward and reinforcement through interaction with dopamine and endogenous opioid signaling. Neuropsychopharmacology. 2018;43:103–15.
Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharm Biochem Behav. 2005;81:263–84.
Author information
Authors and Affiliations
Contributions
EG, ELG, and ZXX designed the experiments. EG, GHB, AM, KC, and YH conducted the experiments. EG, GHB, and ZXX performed data analyses. EG and ZXX wrote the manuscript. All authors have approved the final version of this article.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Galaj, E., Bi, GH., Moore, A. et al. Beta-caryophyllene inhibits cocaine addiction-related behavior by activation of PPARα and PPARγ: repurposing a FDA-approved food additive for cocaine use disorder. Neuropsychopharmacol. 46, 860–870 (2021). https://doi.org/10.1038/s41386-020-00885-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-020-00885-4
This article is cited by
-
New designer phenethylamines 2C-C and 2C-P have abuse potential and induce neurotoxicity in rodents
Archives of Toxicology (2021)