Abstract
Interhemispheric connections across the corpus callosum have a predominantly inhibitory effect. Previous electrophysiology studies imply that local inhibitory circuits are responsible for inducing transcallosal inhibition, likely through inhibitory GABAB-mediated neurotransmission. We investigated the neurochemical mechanisms involved in interhemispheric connectivity by measuring transcranial magnetic stimulation (TMS)-induced interhemispheric signal propagation (ISP) in the motor cortex and dorsolateral prefrontal cortex (DLPFC) with electroencephalography (EEG) recordings under the pharmacological effects of baclofen, l-DOPA, dextromethorphan, and rivastigmine. We hypothesized that for both stimulated regions, GABAB receptor agonist baclofen would decrease ISP when compared against baseline while drugs that target other neurotransmitter systems (dopaminergic, acetylcholinergic, and glutamatergic systems) would have no effect on ISP. Twelve right-handed healthy volunteers completed this study and underwent TMS across five sessions in a randomized order. In the motor cortex, participants showed a significant decrease in ISP under baclofen, but not in the other drug conditions. There were no drug-induced changes in ISP in the DLPFC and baseline ISP did not differ across experimental sessions for both brain regions. Together, our results suggest that the inhibitory effects observed with interhemispheric signal transmission are mediated by a population of interneurons involving GABAB receptor neurotransmission. Inhibitory mechanisms of ISP may be more salient for motor-related functions in the motor cortex than for cognitive control in the DLPFC. These findings are a fundamental step in advancing our understanding of interhemispheric connectivity and may be used to identify treatments for disorders in which transcallosal transmission is dysfunctional.
Similar content being viewed by others
Introduction
As the largest white matter tract in the brain, the corpus callosum plays a major role in controlling the exchange of information between homologous cortical regions in contralateral hemispheres [1]. This pathway facilitates the lateralization of particular brain functions, including spatial processing and facial recognition in the right hemisphere, linguistic processes in the left hemisphere, and motor hand dominance controlled by the contralateral hemisphere. Relating to ideas of metacontrol, signal processing confined to a single dominant hemisphere prevents maladaptive cross-talk between hemispheres for lateralized functions to be carried out with greater efficiency [2, 3]. For motor function, suppression of signal propagation to the ipsilateral motor cortex is an essential physiological mechanism for unilateral hand movements [4, 5]. Otherwise, impaired interhemispheric transmission can result in the occurrence of mirrored bilateral movements, which have been implicated in callosal agenesis [4], schizophrenia [6], and stroke [7], all which involve structural callosal abnormalities. In the dorsolateral prefrontal cortex (DLPFC), corpus callosal fibres play a primary role in cognition. A growing body of literature indicates that structural deficits in callosal fibres correlate with cognitive dysfunction in neurodegenerative disorders, including autism [8], schizophrenia [9], and attention deficit-hyperactive disorder [10].
Neuronal axons mediating interhemispheric communication travel through the corpus callosum to exert their inhibitory effects contralaterally. Since callosal fibres are mostly excitatory in nature [11, 12], inhibitory transmission is likely achieved through the disynaptic activation of local inhibitory circuits [13, 14]. Electrophysiological recordings combined with pharmacology have provided evidence for the direct involvement of GABA receptor subtypes in generating these inhibitory transcallosal potentials [11]. Both GABAA and GABAB receptor antagonists increase the number of spikes contralaterally and shorten the latency of transcallosal stimulation [15]. However, only GABAB antagonists attenuate presynaptic inhibition at callosal synapses when measured through a paired-pulse stimulation paradigm [16]. These results verify the GABAergic regulation of callosal neurotransmission and imply that a GABAB component occurs presynaptically at excitatory callosal terminals. Studying the mechanisms involved in GABABergic neurotransmission could advance our understanding of interhemispheric connectivity while helping to identify treatments for disorders in which callosal transmission is dysfunctional.
Transcranial magnetic stimulation (TMS) combined with electroencephalography (EEG) provides a cause-and-effect approach to investigate the physiological properties of cortico-cortical connectivity non-invasively [17, 18]. TMS-evoked interhemispheric signal propagation (ISP) measures the transmission of cortical-evoked activity between contralateral hemispheres and reliably indices interhemispheric activity across the DLPFC and motor cortex [19]. Furthermore, ISP correlates with the microstructural integrity of callosal genu and motor fibres and demonstrates neuroanatomical specificity with these relationships [19]. There are several lines of TMS evidence implying that ISP relies on GABAergic circuits. Interhemispheric inhibition (IHI), a measure of transcallosal cortico-cortical inhibition that leads to a reduction in the TMS-evoked motor response, inhibits short interval intracortical inhibition), a measure of GABAA receptor activity, and is reduced in the presence of GABAB-mediated long interval intracortical inhibition [20]. These results are consistent with the role of presynaptic GABAB receptors in inhibiting GABA release [21] and imply that IHI involves GABAB-mediated neurotransmission. The pharmacological basis of IHI has not been conclusively established [22, 23]. A major advantage of using ISP over IHI as a TMS index of interhemispheric connectivity is that cortical activity can be measured in both motor and non-motor regions, including the DLPFC. TMS-EEG indexing from the DLPFC holds more relevance to human cognition and neuropsychiatric disorders [24].
The objective of this study was to investigate the neurochemical basis of ISP using a pharmacological agent that positively modulates GABAB receptor activity (baclofen). To our knowledge, the effect of GABAB receptor modulation on interhemispheric connectivity has not been investigated using ISP as an outcome measure. We aimed to demonstrate the specificity of the effects of baclofen on ISP by comparing it against other drugs (l-DOPA, rivastigmine, and dextromethorphan) that target other neurotransmitter systems (dopaminergic, cholinergic, and glutamatergic systems, respectively). We utilized and reanalysed our previously published data [25], which indicated that cortical inhibition in the DLPFC could be pharmacologically modulated by GABABergic and cholinergic agents. These drugs were selected because they have been used to modulate intracortical inhibition in previous studies [25,26,27,28]. Consistent with the role of the corpus callosum in maintaining laterality in motor and prefrontal regions through local inhibitory circuits, we hypothesized that for both brain areas: (1) only baclofen would modulate interhemispheric activity when compared against baseline (2) ISP would be reliably elicited for each participant across baseline.
Methods
Study design
This was a double-blinded randomized placebo-controlled within-subject crossover study. Subjects participated in five sessions whereby 100 single pulses of TMS were applied to the left motor cortex and then 100 pulses to the left DLPFC. To ensure that the researchers and participants were blinded to drug assignment, the Centre for Addiction and Mental Health Pharmacy was responsible for designating alphabetical codes for each drug condition and generating a randomization table to schedule drug administration for all subjects. Following this table, researchers administered placebo or one of the four active drugs at a time when peak plasma levels would be reached (1 h for baclofen, 3 h for dextromethorphan, 1 h for l-DOPA, 2 h for rivastigmine) during post-drug TMS measures [25]. Placebo was randomly given to each participant at 1, 2, or 3 h prior to post-drug administration of TMS, covering all times for active drug plasma peaks. Drug dosages (50 mg of baclofen, 150 mg of dextromethorphan, 100 mg of l-DOPA, 3 mg of rivastigmine) and time points of post-drug measurements were selected based on previous studies demonstrating an effect on cortical inhibition in the motor cortex with these parameters [29,30,31,32,33]. Each session was separated by at least 1 week to exclude carryover effects. The experiments and data analyses were completed while blinded to drug assignment [25] and researchers were only unblinded upon finalization of the study.
Participants
Thirteen healthy participants (mean age 31.3 ± 10.5 years, range 18–55 years, four females) participated in this study after providing written informed consent. Eligibility criteria included no diagnosis of any neurological or psychiatric disorder, no medication usage during or up to 2 weeks before participating in the study, non-smokers, right-handedness to ensure homogeneity of hemisphere dominance, and no contraindication to TMS [34] or MRI. Urine screening was performed to ensure participants tested negative for drugs of abuse and pregnancy for female participants. This study was conducted in accordance with ethical standards of the responsible committee on human experimentation and approved by the Centre for Addiction and Mental Health Research Ethics Board. All participants completed all sessions except for one participant who dropped out after one session. Data for this participant were not included in the analyses.
TMS-EEG in the motor cortex and DLPFC
Single monophasic TMS pulses were administered to the left motor cortex and left DLPFC using a 7-cm figure-of-eight coil and a Magstim 200 stimulator (Magstim Company, Carmarthenshire, Wales). The participant’s resting motor threshold (RMT) was defined as the minimum stimulus intensity to elicit motor-evoked potentials (MEPs) greater than 50 μV in 5 out of 10 trials in the abductor pollicis brevis (APB) muscle [35]. MEP was measured through electromyography (EMG) recordings in the APB muscle. RMT was established by stimulating an optimal location over the left motor hand area, which typically corresponded to the C3 electrode on the international 10–20 EEG system [36]. The RMT stimulus intensity was then adjusted to suprathreshold intensity (~120% RMT) with a mean peak-to-peak amplitude of 1 mV over 20 trials. This intensity was used to deliver 100 single pulses to the left motor cortex and 100 single pulses to the left DLPFC pre-drug and post-drug to assess changes in ISP [25].
Stimulation of the motor cortex was localized to the left motor hand area, as established through RMT procedures. To localize the DLPFC, each participant’s T1-weighted MRI was co-registered to participants’ heads using a magnetic tracking device (miniBIRD system, Ascension Technology Group). Stimulation of the left DLPFC was targeted at the Talairach coordinates x, y, z = −50, 30, 36. This corresponds to the posterior area of Brodmann area (BA) 9 and the superior section of BA 46, based on a conservative definition of these areas from previous research [37]. The handle of the TMS coil was pointed approximately 45° to the midsagittal line during left DLPFC stimulation. Neuronavigation methods ensured identical placement and orientation of the TMS coil within- and between-experiment sessions [25, 38].
EEG was performed using a 64-channel Synamps 2 EEG system (Compumedics, Charlotte, North Carolina) using a 64-channel EEG cap. The impedance of all electrodes (Ag/AgCl ring electrodes) was lowered to ≤5 kΩ and re-referenced to an electrode positioned posterior to the CZ electrode. In addition, four electrodes were placed on the outer corner of each eye, as well as above and below the left eye, to monitor the eye movement artefact. EEG signals were recorded with DC using a lowpass filter, anti-aliasing filter of 200 Hz, at 20 kHz sampling rate, which has been demonstrated to avoid amplifier saturation and minimize the TMS-related artefact [39].
EEG data processing
EEG recordings were processed offline using Neuroscan (Compumedics, Charlotte, North Carolina) and downsampled from 20 to 1 kHz. Analysis of EEG data was performed using EEGLAB [40] and a custom-made script developed in MATLAB (R2016a; The MathWorks Natick, MA, USA). Data were segmented into epochs (−2000 to 2000 ms) around the TMS pulse and baseline corrected using the mean of the TMS artefact-free time period (−500 to −10 ms). To minimize the TMS-related amplifier ringing artefact, data around the TMS pulse (−2 to 10 ms) were removed and linearly interpolated [41, 42]. Thereafter each trial was visually scrutinized and trials containing excessive artefacts due to eye movements or muscle activation were eliminated [43]. Contaminated electrodes were manually removed and interpolated by its nearest neighbours. EEG data were digitally filtered using a second-order, Butterworth, 58–62 Hz notch filter, followed by a fourth-order, Butterworth, zero-phase shift 1–55 Hz band pass filter [44]. Next, EEG data underwent a first round of independent component analysis (EEGLAB toolbox) to detect and remove TMS decay artefacts and high amplitude muscle artefacts [45]. Pre- and post-drug conditions were concatenated together and underwent a second round of ICA to apply the same objective criteria when de-noising the data from other artefacts (i.e. eye blinks, eye movements, muscle artefacts) [44]. Finally, data were re-referenced to the average to generate a clean signal for each participant.
TMS-induced ISP
ISP was quantified by calculating the ratio of TMS-evoked potentials (TEPs) in the right cortex over the left cortex [19]. In the motor cortex, ISP was calculated using the averaged-trial rectified TEP curve from the C3 (closest electrode to left motor hand region) and C4 electrode (corresponding electrode to the right motor region). In the DLPFC, the recording electrodes of interest were F5 (left hemisphere) and F6 (right hemisphere), in accordance with the Talairach coordinates targeted during neuronavigation. For the left (stimulated) hemisphere, the area under the curve was obtained between 50 and 150 ms post-stimulus, with respect to the earliest onset of guaranteed artefact-free data [19]. We selected an average interhemispheric transfer time of 10 ms [46, 47]. For the right (unstimulated) hemisphere, a time window of 60–160 ms was used, in accordance with previous methods [19, 48, 49].
Statistical analysis
All statistical analyses were performed using SPSS 16.0 (SPSS, Chicago, Illinois). Data were first checked for normality using the Kolmogorov–Smirnov test (p > 0.05). To test our primary hypothesis whether there is a specific baclofen-induced effect on ISP for both the motor cortex and DLPFC, planned comparisons between pre-drug vs. post-drug ISP values were performed using multiple dependent sample t-tests for each drug condition. Bonferroni correction was applied for the different drug groups (n = 5) and cortical regions (n = 2) investigated, reflected by a corrected critical α value of 0.005. Effect sizes (d′) were evaluated with Cohen’s d and are reported for these comparisons. To assess whether baseline ISP measurements are similar and reproducible in the five pre-drug conditions, a repeated measures analysis of variance (rmANOVA) was employed with drug as the within-subject factor. Cronbach’s alpha, a widely accepted measure of reproducibility in EEG studies [50, 51], was also used to assess test–retest reliability of ISP across baseline. All data are presented as mean ± SEM unless otherwise noted.
Results
All outcome data were normally distributed. Across all testing days and drug groups, 1 mV and RMT stimulation intensities did not differ. Demographic and neurophysiological characteristics of all participants are presented in Table 1.
Drug-induced modulation of ISP
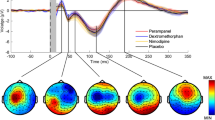
Baclofen was the only drug that affected ISP in the motor cortex, leading to a significant reduction in ISP even after Bonferroni correction (Fig. 1a, Table 2). To verify this effect, we compared the change in ISP between the baclofen and placebo groups, which also yielded significance (t(11) = 2.267, p = 0.045). We also compared the pre- vs post-baclofen levels of cortical-evoked activation in the left motor cortex to ensure that the observed changes in ISP were not confounded by alterations within the stimulated hemisphere alone. Cortical-evoked activity (50–150 ms post-TMS) in the left motor cortex did not differ after the administration of baclofen (t(11) = 1.497, p = 0.163). Additionally, we investigated the baclofen-induced change in ISP at the level of individual subjects. The majority of subjects (11 out of 12 subjects) exhibited decreases in ISP after the intake of baclofen, while only 1 subject displayed an opposite effect (Fig. 1b). Butterfly plots and the topography of surface voltage TEPs at different time points are shown in Fig. 1c, d.
Baclofen reduces ISP in the motor cortex. a Line plots of the ISP group averages for the five drug conditions (placebo, l-DOPA, baclofen, rivastigmine, dextromethorphan) across time (pre, post). b Scatter plots for the pharmacological modulation of ISP at the level of individual subjects, expressed as post-drug minus pre-drug. Error bars represent group mean ± SEM. c Butterfly plots contain TEP traces from all electrodes in the pre-baclofen and post-baclofen conditions (grand-averaged across participants). Red trace highlights the TEP from the left motor cortex electrode (C3) and blue trace highlights the TEP from the right motor cortex electrode (C4). d Topoplots illustrate the distribution of cortical-evoked activity across all electrodes at different time points in the pre-baclofen and post-baclofen conditions (grand-averaged across participants). Crosses indicate the site of TMS stimulation over the C3 electrode. Open circles indicate the location of the C4 electrode
No significant changes in ISP were found in the DLPFC after administration of l-DOPA, baclofen, rivastigmine, dextromethorphan, or placebo (Fig. 2a, Table 2). At the level of individual subjects, only 8 out of 12 subjects demonstrated decreases in ISP after baclofen (Fig. 2b), suggesting reduced consistency of the effects of baclofen on DLPFC ISP compared to motor ISP.
No effect of drugs on ISP in the DLPFC. a Line plots of the ISP group averages for the five drug conditions (baclofen, dextromethorphan, l-DOPA, placebo, rivastigmine) across time (pre, post). b Scatter plot of the pharmacological modulation of ISP at the level of individual subjects, expressed as post-drug minus pre-drug. Horizontal bars represent group mean ± SEM
Replicating TMS-induced ISP
Mauchly’s test was used to verify that all data met the assumptions of sphericity required for the rmANOVA. In the motor cortex, a one-way rmANOVA revealed no significant effect of time on the mean TMS-induced ISP at baseline (F(4,40) = 0.67, p = 0.62) (Fig. 3). Cronbach’s α (value = 0.86) revealed a high level of reproducibility for ISP across all drug conditions.
Similarly in the DLPFC, a one-way rmANOVA found no significant differences between pre-drug ISP values (F(4,40) = 0.35, p = 0.85) (Fig. 3). Cronbach’s α (value = 0.68) falls just short of the critical value (0.70), suggesting that ISP in the DLPFC can be elicited with moderate-high reliability as well.
Discussion
This study confirmed our primary hypothesis that baclofen, a GABAB receptor agonist, reduces ISP in vivo in response to motor cortex stimulation. Furthermore, we demonstrated that baseline ISP is a reproducible measure of interhemispheric activity in the motor and prefrontal regions over time. We did not demonstrate that baclofen modulates ISP from DLPFC stimulation. To our knowledge, this is the first study to assess pharmacological modulation of ISP in both the motor and prefrontal cortices.
Our first confirmed hypothesis was that baclofen reduces motor cortical ISP. This finding is consistent with electrophysiology studies in animals demonstrating increased IHI after focal application of baclofen [52], attenuated IHI after application of GABAB antagonist CGP52432 [16, 53], and elimination of IHI in transgenic mice lacking GABAB receptors [52]. As mentioned previously, IHI measures the ratio of inhibition between the conditioned and unconditioned MEP, while ISP measures the ratio of cortical-evoked activity between contralateral cortices. Hence, increased IHI from positive GABAB modulation is in line with our finding of reduced ISP from baclofen. Previous TMS studies were not able to establish a modulatory effect of baclofen in motor cortical IHI [22, 23]. Our result suggests that ISP may be a more sensitive measure of interhemispheric activity to pharmacological modulation than IHI, perhaps because ISP directly probes cortico-cortical connectivity while IHI relies on peripheral motor mechanisms. Future studies should explore whether baclofen influences both ISP and IHI in the same subject group to directly investigate the relationship between these two measures. We also confirmed that motor thresholds and stimulation intensities were unchanged between experimental sessions. This helps confirm that changes in ISP arose from pharmacological intervention rather than methodological differences. In addition, baclofen was the only drug we tested that led to consistent effects on individual subject ISP values. Overall, these findings corroborate the role of GABAB receptor-mediated neurotransmission in mediating ISP across the motor cortices.
ISP following left DLPFC stimulation showed a non-significant decrease after baclofen (p = 0.20). Although we assumed that interhemispheric connections between the prefrontal and motor cortices operated under similar mechanisms, this finding suggests that inhibitory ISP may be more salient in motor function than for cognitive control. Our speculation is supported by physiological evidence indicating that highly lateralized motor cortex functions require IHI to coordinate motor movements and produce a unified motor response [4]. Meanwhile, in the DLPFC, more efficient interhemispheric connectivity is generally associated with greater cognitive performance [54, 55], presumably because the corpus callosum facilitates the sharing of hemispheric resources for cognitive integration [1]. In contrast to the largely inhibitory connections that exist between motor cortices, our findings suggest that there may be a greater balance between inhibition and excitation in the DLPFC. To our knowledge, the neurochemical properties of genu signal transmission have not been directly investigated in humans. Recent evidence from animal studies imply that callosal communication between prefrontal regions involves complex interactions between glutamate, GABA, and dopamine-sensitive mechanisms [56, 57]. In our study, we did not see a significant change in ISP when we pharmacologically modulated these different transmitter systems. This reflects the complexity of interhemispheric DLPFC projections for functional cognitive processes which may involve a diversity of synaptic inputs from different neuromodulatory and transmitter systems.
Our second finding was that ISP demonstrates high test–retest reliability and internal consistency in both the prefrontal and motor cortices. This replicates a previous TMS-EEG study that reported high Cronbach’s alpha scores for ISP in both cortical regions [19] and further confirms that TMS-EEG is a valid and reliable neurophysiological technique to probe interhemispheric connectivity in the motor cortex and DLPFC. Other TMS markers of interhemispheric signal processes show variable reproducibility between subjects across separate experimental sessions [58, 59]. A likely explanation is that these indices measure a reduction in MEP amplitude, which is inherently variable due to its dependence on corticospinal pathways [60]. Furthermore, variation in the placement and orientation of the TMS coil between and within experimental sessions may account for some of these observed differences. We conducted TMS-EEG with neuronavigation procedures to help ensure identical and consistent placement of the TMS coil against the targeted cortical region. Our demonstration of high ISP reproducibility strengthens our primary finding that ISP can be used to reliably evaluate inhibitory interhemispheric connectivity between contralateral motor cortices. This circuitry may be more complex in the DLPFC and requires further investigation.
We showed that interhemispheric connectivity can be effectively modulated using a GABAB receptor agonist. Did baclofen exert its inhibitory effect on ISP by targeting presynaptic callosal projection neurons or by inducing an inhibitory potential upon postsynaptic targets? Since baclofen does not selectively act on particular subunits of the GABAB receptor, both effects can be expected [61, 62]. Recent animal studies imply that callosal inhibition is reliant on postsynaptic GABABergic neurotransmission and accordingly, that baclofen acts upon these extrasynaptic GABAB receptors located on pyramidal apical dendrites in the unstimulated hemisphere [52, 63]. However, the disinhibitory effect on IHI produced by paired-pulse TMS stimulation is consistent with those mechanisms mediated by presynaptic GABAB receptors [16]. Additionally, some callosal projection neurons are GABAergic in nature [64, 65], and we cannot exclude the possibility that these neurons were directly targeted by baclofen to magnify the inhibitory effects seen contralaterally. In support of this hypothesis, a previous TMS-EEG study found that baclofen increases the amplitude of the N100, a marker of GABAB receptor activity, selectively in the stimulated hemisphere [28]. In our data, only the ratios of right over left cortical-evoked activity, and not the level of activation in the left hemisphere itself, were significantly different after baclofen. Although we could not verify with the present experiment whether baclofen induced hemisphere-specific and receptor subtype-specific changes, we speculate that the reduction of ISP through baclofen was mediated through an interaction of both presynaptic and postsynaptic effects at the level of local inhibitory circuits.
Impaired interhemispheric communication is implicated in the pathology of a number of neurological disorders, including callosal agenesis, epilepsy, stroke, and multiple sclerosis, as well as neuropsychiatric disorders, including depression, schizophrenia, and autism spectrum disorder (for review, see [66]). As these deficits are typically accompanied by microstructural alterations in the corpus callosal fibres, TMS-EEG offers a unique opportunity to non-invasively investigate impaired interhemispheric connectivity with ISP as an index. However, few studies to date have investigated clinical alterations in ISP. No differences in ISP for motor areas were reported between healthy controls and patients with early multiple sclerosis [48] or autism spectrum disorder [49], potentially due to a wide clinical heterogeneity within the patient samples. Although ISP has never been assessed in patients with schizophrenia, abnormal IHI is consistently demonstrated in both medicated and unmedicated chronic schizophrenia patients [67, 68]. Excessive motor overflow, resulting from disinhibitory interhemispheric deficits, may contribute to motor dysfunction in schizophrenia. TMS-EEG research also implicates frontal interhemispheric asymmetry as a biomarker of depression [69] and restoring this asymmetry with repetitive TMS treatment has been found to ameliorate depressive symptoms [70, 71]. Since ISP can be measured with high reliability from both motor and prefrontal regions, it would be of great interest to assess potential disruption of ISP in cortical regions implicated in the pathophysiology of schizophrenia and depression.
This exploratory study is limited by the relatively small sample size. As we performed secondary analyses upon pharmaco-TMS data that was initially measuring intracortical inhibition [25], our small sample may have prevented the detection of weaker pharmacological effects, particularly in the DLPFC. However, the large effect size of our pre- vs. post-baclofen comparison in the motor cortex (d′ = 1.050) suggests that we are sufficiently powered to investigate these effects on ISP, as per our a priori hypothesis. Additionally, the TEPs may have been contaminated by sensory-evoked potentials (SEPs) occurring during stimulation [72, 73]. SEPs have been shown to impact signal in both left (stimulated) hemisphere electrodes and right (unstimulated) hemisphere electrodes [73]. To reduce the overall contribution of SEPs to ISP, we calculated ISP as a ratio of right over left hemisphere cortical-evoked activation. Additionally, we utilized suprathreshold stimulation intensities which have the advantage of evoking neural responses that are much larger than multisensory ones, thus leading to a higher signal-to-noise ratio [74], although this may have also increased the risk for sensory contamination. We replicated the same methodology before and after drug delivery to minimize the effects of auditory and somatosensory-evoked artefacts on our group comparisons. Finally, the issue of a potential SEP influence on ISP has been addressed previously, and ISP was shown to be preserved even after evoked potentials from sham stimulation were subtracted from those of active suprathreshold TMS stimulation [19].
In conclusion, we used a pharmaco-TMS approach to assess the drug-induced modulation of interhemispheric communication across the corpus callosum. Our data demonstrate that ISP from motor cortex stimulation is reduced via GABABergic neurotransmission. Future work should assess the precise neurobiological mechanisms by which GABABergic agents act upon to modulate ISP and whether this can be replicated across different measures of interhemispheric connectivity, such as EEG resting state functional connectivity. These analyses may have important implications for our understanding of drug-induced changes in cognitive and motor processing [75,76,77]. Additionally, it would be of great interest to directly verify TMS-induced signal propagation between contralateral homotopic brain regions using source analysis. Lastly, our finding of high reproducibility of ISP across time suggests that TMS-EEG can also be used to study transcallosal activity in clinical populations in which abnormal intercortical inhibition has been implicated.
Funding and disclosure
J.H. was supported by the Centre for Addiction and Mental Health (CAMH) Discovery Fund studentship award and the Ontario Graduate Scholarship (OGS). P.L. has received consultation fees from Nexstim Ltd. (Helsinki Finland) for purposes unrelated to the present study. T.K.R. has received research support from Brain Canada, Brain and Behavior Research Foundation, BrightFocus Foundation, Canada Foundation for Innovation, Canada Research Chair, Canadian Institutes of Health Research, Centre for Aging and Brain Health Innovation, National Institutes of Health, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, and the Weston Brain Institute. T.K.R. also received in-kind equipment support for an investigator-initiated study from Magstim. D.M.B. has received research support from CIHR, NIH, Brain Canada and the Temerty Family through the CAMH Foundation and the Campbell Research Institute. He receives research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. and he is the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. D.M.B. also receives in-kind equipment support from Magventure for an investigator-initiated study. He receives medication supplies for an investigator-initiated trial from Indivior. In the last 5 years, Z.J.D. has received research and equipment in-kind support for an investigator-initiated study through Brainsway Inc. and Magventure Inc. His work was supported by the Ontario Mental Health Foundation (OMHF), the Canadian Institutes of Health Research (CIHR), the National Institutes of Mental Health (NIMH), and the Temerty Family and Grant Family and through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Institute. J.H., R.Z., P.L., B.S., and R.C. declare no biomedical interests or conflicts.
References
Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev. 2005;15:59–71.
Banich MT, Belger A. Interhemispheric interaction: how do the hemispheres divide and conquer a task? Cortex. 1990;26:77–94.
Banich MT. Interhemispheric processing: theoretical considerations and empirical approaches. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. Cambridge, MA: MIT Press; 1995. p. 427–50.
Mayston MJ, Harrison LM, Stephens JA. A neurophysiological study of mirror movements in adults and children. Ann Neurol. 1999;45:583–94.
Kicić D, Lioumis P, Ilmoniemi RJ, Nikulin VV. Bilateral changes in excitability of sensorimotor cortices during unilateral movement: combined electroencephalographic and transcranial magnetic stimulation study. Neuroscience. 2008;152:1119–29.
Aydin K, Ucok A, Guler J. Altered metabolic integrity of corpus callosum among individuals at ultra high risk of schizophrenia and first-episode patients. Biol Psychiatry. 2008;64:750–7.
Nelles G, Cramer SC, Schaechter JD, Kaplan JD, Finklestein SP. Quantitative assessment of mirror movements after stroke. Stroke. 1998;29:1182–7.
Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–6.
Swayze VW, Andreasen NC, Ehrhardt JC, Yuh WT, Alliger RJ, Cohen GA. Developmental abnormalities of the corpus callosum in schizophrenia. Arch Neurol. 1990;47:805–8.
Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D, Lyytinen H. Corpus callosum morphology in attention deficit-hyperactivity disorder: morphometric analysis of MRI. J Learn Disabil. 1991;24:141–6.
Kawaguchi Y. Receptor subtypes involved in callosally-induced postsynaptic potentials in rat frontal agranular cortex in vitro. Exp Brain Res. 1992;88:33–40.
Voigt T, LeVay S, Stamnes MA. Morphological and immunocytochemical observations on the visual callosal projections in the cat. J Comp Neurol. 1988;272:450–60.
Toyama K, Matsunami K, Ono T, Tokashiki S. An intracellular study of neuronal organization in the visual cortex. Exp Brain Res. 1974;21:45–66.
Naito H, Nakamura H, Kurosaki T, Tamura Y. Transcallosal excitatory postsynaptic potentials of fast and slow pyramidal tract cells in cat sensorimotor cortex. Brain Res. 1970;19:299–301.
Chowdhury SA, Kawashima T, Konishi T, Matsunami K. GABAergic characteristics of transcallosal activity of cat motor cortical neurons. Neurosci Res. 1996;26:323–33.
Chowdhury SA, Matsunami KI. GABA-B-related activity in processing of transcallosal response in cat motor cortex. J Neurosci Res. 2002;68:489–95.
Ilmoniemi RJ, Ruohonen J, Karhu J. Transcranial magnetic stimulation—a new tool for functional imaging of the brain. Crit Rev Biomed Eng. 1999;27:241–84.
Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32.
Voineskos AN, Farzan F, Barr MS, Lobaugh NJ, Mulsant BH, Chen R, et al. The role of the corpus callosum in transcranial magnetic stimulation induced interhemispheric signal propagation. Biol Psychiatry. 2010;68:825–31.
Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543(Pt 1):317–26.
Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530(Pt 2):307–17.
Irlbacher K, Brocke J, Mechow Jv, Brandt SA. Effects of GABAA and GABAB agonists on interhemispheric inhibition in man. Clin Neurophysiol. 2007;118:308–16.
Müller-Dahlhaus JFM, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 2008;586:495–514.
Tremblay S, Rogasch NC, Premoli I, Blumberger DM, Casarotto S, Chen R, et al. Clinical utility and prospective of TMS–EEG. Clin Neurophysiol. 2019;130:802–44.
Salavati B, Rajji TK, Zomorrodi R, Blumberger DM, Chen R, Pollock BG, et al. Pharmacological manipulation of cortical inhibition in the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2018;43:354–61.
Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51:1320–4.
Priori A, Berardelli A, Inghilleri M, Accornero N, Manfredi M. Motor cortical inhibition and the dopaminergic system. Pharmacological changes in the silent period after transcranial brain stimulation in normal subjects, patients with Parkinson’s disease and drug-induced parkinsonism. Brain. 1994;117(Pt 2):317–23.
Premoli I, Castellanos N, Rivolta D, Belardinelli P, Bajo R, Zipser C, et al. TMS-EEG signatures of GABAergic neurotransmission in the human cortex. J Neurosci. 2014;34:5603–12.
McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93.
Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543(Pt 2):699–708.
Crevoisier C, Hoevels B, Zürcher G, Da Prada M. Bioavailability of L-Dopa after Madopar HBS administration in healthy volunteers. Eur Neurol. 1987;27:36–46.
Kuo M-F, Paulus W, Nitsche MA. Boosting focally-induced brain plasticity by dopamine. Cereb Cortex. 2008;18:648–51.
Kuo M-F, Grosch J, Fregni F, Paulus W, Nitsche MA. Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J Neurosci. 2007;27:14442–7.
Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMS Consensus Group TS of TC. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39.
Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92.
Herwig U, Satrapi P, Schönfeldt-Lecuona C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16:95–9.
Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–22.
Salavati B, Daskalakis ZJ, Zomorrodi R, Blumberger DM, Chen R, Pollock BG, et al. Pharmacological modulation of long-term potentiation-like activity in the dorsolateral prefrontal cortex. Front Hum Neurosci. 2018;12:155.
Rajji TK, Sun Y, Zomorrodi-Moghaddam R, Farzan F, Blumberger DM, Mulsant BH, et al. PAS-induced potentiation of cortical-evoked activity in the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2013;38:2545–52.
Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21.
Bergmann TO, Mölle M, Schmidt MA, Lindner C, Marshall L, Born J, et al. EEG-guided transcranial magnetic stimulation reveals rapid shifts in motor cortical excitability during the human sleep slow oscillation. J Neurosci. 2012;32:243–53.
Rogasch NC, Sullivan C, Thomson RH, Rose NS, Bailey NW, Fitzgerald PB, et al. Analysing concurrent transcranial magnetic stimulation and electroencephalographic data: a review and introduction to the open-source TESA software. Neuroimage. 2017;147:934–51.
Rogasch NC, Thomson RH, Daskalakis ZJ, Fitzgerald PB. Short-latency artifacts associated with concurrent TMS–EEG. Brain Stimul. 2013;6:868–76.
Voineskos D, Blumberger DM, Zomorrodi R, Rogasch NC, Farzan F, Foussias G, et al. Altered transcranial magnetic stimulation-electroencephalographic markers of inhibition and excitation in the dorsolateral prefrontal cortex in major depressive disorder. Biol Psychiatry. 2018;85:477–86.
Rogasch NC, Thomson RH, Farzan F, Fitzgibbon BM, Bailey NW, Hernandez-Pavon JC, et al. Removing artefacts from TMS-EEG recordings using independent component analysis: importance for assessing prefrontal and motor cortex network properties. Neuroimage. 2014;101:425–39.
Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–46.
Meyer BU, Röricht S, Gräfin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118(Pt 2):429–40.
Zipser CM, Premoli I, Belardinelli P, Castellanos N, Rivolta D, Heidegger T, et al. Cortical excitability and interhemispheric connectivity in early relapsing-remitting multiple sclerosis studied with TMS-EEG. Front Neurosci. 2018;12:393.
Jarczok TA, Fritsch M, Kröger A, Schneider AL, Althen H, Siniatchkin M, et al. Maturation of interhemispheric signal propagation in autism spectrum disorder and typically developing controls: a TMS-EEG study. J Neural Transm. 2016;123:925–35.
Kondacs A, Szabó M. Long-term intra-individual variability of the background EEG in normals. Clin Neurophysiol. 1999;110:1708–16.
Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB, et al. Reliability of long-interval cortical inhibition in healthy human subjects: ATMS–EEG Study. J Neurophysiol. 2010;104:1339–46.
Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME. The cellular basis of GABA(B)-mediated interhemispheric inhibition. Science. 2012;335:989–93.
Kokinovic B, Medini P. Loss of GABAB-mediated interhemispheric synaptic inhibition in stroke periphery. J Physiol. 2018;596:1949–64.
Chaddock-Heyman L, Erickson KI, Voss MW, Powers JP, Knecht AM, Pontifex MB, et al. White matter microstructure is associated with cognitive control in children. Biol Psychol. 2013;94:109–15.
Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen N-K, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822:386–400.
Murakami G, Nakamura M, Takita M, Ishida Y, Ueki T, Nakahara D. Brain rewarding stimulation reduces extracellular glutamate through glial modulation in medial prefrontal cortex of rats. Neuropsychopharmacology. 2015;40:2686–95.
Lupinsky D, Moquin L, Gratton A. Interhemispheric regulation of the medial prefrontal cortical glutamate stress response in rats. J Neurosci. 2010;30:7624–33.
De Gennaro L, Ferrara M, Bertini M, Pauri F, Cristiani R, Curcio G, et al. Reproducibility of callosal effects of transcranial magnetic stimulation (TMS) with interhemispheric paired pulses. Neurosci Res. 2003;46:219–27.
Fleming MK, Newham DJ. Reliability of transcallosal inhibition in healthy adults. Front Hum Neurosci. 2017;10:681.
Amassian VE, Cracco RQ, Maccabee PJ. Focal stimulation of human cerebral cortex with the magnetic coil: a comparison with electrical stimulation. Electroencephalogr Clin Neurophysiol Potentials Sect. 1989;74:401–16.
Mott DD, Lewis DV. The pharmacology and function of central GABAB receptors. Int Rev Neurobiol. 1994;36:97–223.
Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, et al. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharm Rev. 2002;54:247–64.
Murphy SC, Palmer LM, Nyffeler T, Müri RM, Larkum ME. Transcranial magnetic stimulation (TMS) inhibits cortical dendrites. Elife. 2016;5:e13598.
Clark RM, Collins GG. The release of endogenous amino acids from the rat visual cortex. J Physiol. 1976;262:383–400.
Innocenti GM, Manzoni T, Spidalieri G. Peripheral and transcallosal reactivity of neurons within SI and SII cortical areas. Segmental divisions. Arch Ital Biol. 1972;110:415–43.
van der Knaap LJ, van der Ham IJM. How does the corpus callosum mediate interhemispheric transfer? A review. Behav Brain Res. 2011;223:211–21.
Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002;59:347–54.
Fitzgerald PB, Brown TL, Daskalakis ZJ, deCastella A, Kulkarni J. A study of transcallosal inhibition in schizophrenia using transcranial magnetic stimulation. Schizophr Res. 2002;56:199–209.
Funk AP, George MS. Prefrontal EEG asymmetry as a potential biomarker of antidepressant treatment response with transcranial magnetic stimulation (TMS): a case series. Clin EEG Neurosci. 2008;39:125–30.
Lefaucheur J-P, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125:2150–206.
Kennedy SH, Milev R, Giacobbe P, Ramasubbu R, Lam RW, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. J Affect Disord. 2009;117:S44–53.
ter Braack EM, de Vos CC, van Putten MJAM. Masking the auditory evoked potential in TMS–EEG: a comparison of various methods. Brain Topogr. 2015;28:520–8.
Conde V, Tomasevic L, Akopian I, Stanek K, Saturnino GB, Thielscher A, et al. The non-transcranial TMS-evoked potential is an inherent source of ambiguity in TMS-EEG studies. Neuroimage. 2019;185:300–12.
Belardinelli P, Biabani M, Blumberger DM, Bortoletto M, Casarotto S, David O, et al. Reproducibility in TMS-EEG studies: a call for data sharing, standard procedures and effective experimental control. Brain Stimul. 2019;12:787–90.
Mucci A, Volpe U, Merlotti E, Bucci P, Galderisi S. Pharmaco-EEG in psychiatry. Clin EEG Neurosci. 2006;37:81–98.
Frei E, Gamma A, Pascual-Marqui R, Lehmann D, Hell D, Vollenweider FX. Localization of MDMA-induced brain activity in healthy volunteers using low resolution brain electromagnetic tomography (LORETA). Hum Brain Mapp. 2001;14:152–65.
Drinkenburg WHIM, Ruigt GSF, Ahnaou A. Pharmaco-EEG studies in animals: an overview of contemporary translational applications. Neuropsychobiology. 2015;72:151–64.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hui, J., Zomorrodi, R., Lioumis, P. et al. Pharmacological mechanisms of interhemispheric signal propagation: a TMS-EEG study. Neuropsychopharmacol. 45, 932–939 (2020). https://doi.org/10.1038/s41386-019-0468-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-019-0468-7
This article is cited by
-
Precise Modulation Strategies for Transcranial Magnetic Stimulation: Advances and Future Directions
Neuroscience Bulletin (2021)