Abstract
Second-generation antipsychotic drugs (SGAs) are essential in the treatment of psychotic disorders, but are well-known for inducing substantial weight gain and obesity. Critically, weight gain may reduce life expectancy for up to 20–30 years in patients with psychotic disorders, and prognostic biomarkers are generally lacking. Even though other receptors are also implicated, the dorsal striatum, rich in dopamine D2 receptors, which are antagonized by antipsychotic medications, plays a key role in the human reward system and in appetite regulation, suggesting that altered dopamine activity in the striatal reward circuitry may be responsible for increased food craving and weight gain. Here, we measured striatal volume and striatal resting-state functional connectivity at baseline, and weight gain over the course of 12 weeks of antipsychotic treatment in 81 patients with early-phase psychosis. We also included a sample of 58 healthy controls. Weight measurements were completed at baseline, and then weekly for 4 weeks, and every 2 weeks until week 12. We used linear mixed models to compute individual weight gain trajectories. Striatal volume and whole-brain striatal connectivity were then calculated for each subject, and used to assess the relationship between striatal structure and function and individual weight gain in multiple regression models. Patients had similar baseline weights and body mass indices (BMI) compared with healthy controls. There was no evidence that prior drug exposure or duration of untreated psychosis correlated with baseline BMI. Higher left putamen volume and lower sensory motor connectivity correlated with the magnitude of weight gain in patients, and these effects multiplied when the structure–function interaction was considered in an additional exploratory analysis. In conclusion, these results provide evidence for a correlation of striatal structure and function with antipsychotic-induced weight gain. Lower striatal connectivity was associated with more weight gain, and this relationship was stronger for higher compared with lower left putamen volumes.
Similar content being viewed by others
Introduction
Weight gain is a major side effect of treatment with antipsychotic drugs, but individual weight gain may vary considerably between patients treated with a given antipsychotic agent, especially in the first episode of illness [1].
Although all antipsychotic drugs induce weight gain, some appear to induce more than others [2, 3], and the reasons for the differences between drugs and between patients are unclear [4, 5]. Genetic variability [6,7,8] and lifestyle likely play a role, but structural and functional differences in the brain’s dopaminergic reward system have not been fully understood. In addition to dopamine’s function in food reward and in the control of food intake [9], dopamine is also a key factor in schizophrenia and in antipsychotic action, suggesting that one may expect baseline differences in striatal structure and function to account for the variability in antipsychotic-induced weight gain.
Even though other receptors are also strongly implicated in antipsychotic weight gain [10], the dorsal striatum, rich in dopamine D2 receptors, which are antagonized by antipsychotic medications, plays an important role in the human reward system and in appetite regulation [11,12,13,14], suggesting that altered dopamine activity in the striatal reward circuitry may contribute to increased food craving and the resultant weight gain [15]. In line with this notion, previous work has shown that decreased baseline functional activity in the putamen predicted the amount of future antipsychotic weight gain [16], and olanzapine-induced activity in the dorsal striatum was associated with excessive eating behavior [17]. Supporting the hypothesis of altered reward processing in schizophrenia, these results suggest that decreased reward anticipation in the putamen before antipsychotic treatment may predispose to weight gain under antipsychotic treatment. However, we are not aware of studies in weight gain using risperidone and aripiprazole, two widely used second-generation antipsychotic drugs (SGAs) [18].
Furthermore, in addition to altered activation in fMRI tasks, variability in striatal structure and functional connectivity may also contribute to weight gain. Indeed, previous studies have shown higher striatal volumes in addictive behavior [19, 20], as well as weaker fronto-striatal connectivity in obesity [21]. Thus, we measured striatal volume and striatal resting-state functional connectivity in patients with early-phase psychosis at baseline, and weight gain over the course of 12 weeks of treatment with risperidone and aripiprazole, and hypothesized that higher striatal volume and lower cortico-striatal connectivity would be associated with more weight gain [21, 22], particularly in brain regions implicated in obesity [21, 23]. We also expected that striatal volume would be higher in patients compared with controls, as found in a previous meta-analysis [24].
Materials and methods
Participants
We used two early-phase psychosis cohorts from two separate 12-week clinical trials, with a similar design and similar treatment effects, resulting in a sample of 81 patients. We defined early-phase psychosis as having taken antipsychotic medications for a cumulative lifetime period of 2 years or less. [25, 26]. Details have been published previously [18, 25,26,27] and are summarized in Table 1. Written informed consent was obtained from adult participants and the legal guardians of participants younger than 18 years. All participants under the age of 18 provided written informed assent. The study was approved by the Institutional Review Board (IRB) of Northwell Health. We also included a sample of 58 age-matched healthy controls (Table 1).
Weight assessments and analysis
Weight measurements were completed at baseline, and then weekly for 4 weeks, and every 2 weeks until week 12. Our analysis included the following two steps: We first estimated individual levels of weight gain with linear mixed models, which allowed us to compute individual weight gain trajectories [28]. Specifically, we used weight as the dependent variable and time (measured in days from baseline) as a continuous predictor, and included a random intercept as well as a random slope for day. The random slopes account for the fact that individuals trajectories were allowed to vary around the group mean, thereby capturing the phenomenon of primary interest in our study: individual deviances from the average weight gain trajectory. In a second step, we extracted those random slopes, and used them as dependent variable in a linear multivariable regression, with putamen volume as predictor of interest (see below). In other words, in the second step, we tested our primary hypothesis that it was the putamen volume that was correlated with individual weight gain.
Random slopes were estimated using restricted maximum likelihood and used as the dependent variables in our structural and functional imaging analyses (see below). All analyses were conducted in R version 3.6.0. Data and code of the current study are available online at http://github.com/philipphoman/bmi.
Structural imaging and analysis
Magnetic resonance imaging exams were conducted on a 3-T scanner (GE Signa HDx). We acquired anatomical scans in the coronal plane using an inversion-recovery prepared 3D fast spoiled gradient (IR-FSPGR) sequence (TR = 7.5 ms, TE = 3 ms, TI = 650 ms, matrix = 256 × 256, FOV = 240 mm), which produced 216 contiguous images (slice thickness = 1 mm) through the whole brain. After image processing and segmentation with Freesurfer 5.1.0, we measured the volumes of putamen and caudate and also included the hippocampus as control region.
We then computed multivariable regressions for each region of interest, entering the individual weight gain slopes as the dependent variable and a predictor for subcortical volume. To adjust for unspecific confounders, we included additional variables in these models, namely age, sex, the age by sex interaction, baseline body mass index (BMI), duration of untreated psychosis (DUP), and total intracranial volume.
Since our primary hypothesis was based on the previous observation that the putamen is implicated in antipsychotic weight gain [16], we first tested for an association between the average putamen volume and individual weight gain. After demonstrating this association, we used the same model for the left and right putamen separately. In a second step, we also tested for associations between the caudate and the hippocampus with individual weight gain.
Functional imaging and analysis
We performed preprocessing following recommended procedures [29], after excluding patients with motion of more than two standard deviations above the average framewise displacement (FD) [27]. In total, four participants (one female) were excluded due to excessive head motion. Their demographic variables were inspected and were similar to the patients in the included samples. We processed neuroimaging data using SPM12 and Matlab2015b and applied slice time correction, motion correction, co-registration to the T1-weighted image, segmentation, and normalization. For the segmentation and normalization steps, we segmented the T1-weighted image into six tissue types (gray matter, white matter, cerebrospinal fluid (CSF), air, bone, and background), and used the tissue-probability information to register to the ICBM152 MNI template. We then applied a high-pass temporal filter of 0.005 Hz to all voxel time courses and to the motion time courses before nuisance correction. Nuisances were then regressed out of all voxels using CompCor; this included the 12 motion parameters (absolute and differential motion) and the CompCor components explaining at least 50% of the variance for white matter and CSF voxels [30, 31]. To identify the white matter and CSF voxels that were entered into CompCor, each participant’s white matter and CSF tissue-probability maps were thresholded at >99.8% probability to avoid partial voluming. We performed the analyses both with and without global signal regression. After nuisance regression, functional images were spatially smoothed (6-mm FWHM) and temporally filtered (bandpass 0.01–0.1 Hz). To create connectivity maps, the first time points were removed to allow for signal stabilization. Time courses were then extracted for the putamen seeds, which represented functionally distinct anatomical subregions of the the striatum [32].
Since our structural analysis revealed a single region to be predictive of weight gain, i.e., the left putamen, we used this as a seed region in our functional connectivity analysis. Adopting the approach by Di Martino et al. [32], 4 × 4 × 4-mm spheres were defined in the subregions of the left putamen, including the dorsal rostral putamen (x = −25, y = 8, and z = 6), dorsal caudal putamen (x = −28, y = 1, and z = 3), and ventral rostral putamen (x = −20, y = 12, and z = −3). We computed correlation maps for each participant for all three of our seeds by extracting mean activity time courses from each seed region, and by calculating whole-brain voxelwise correlation maps with the extracted waveform as a reference. The resulting correlation maps were z-transformed.
Mean framewise displacement was also included to control for the residual effect of head motion. Given that use of the data scrubbing to eliminate motion-related artifact offers little advantage over group-level corrections and can correct the data incompletely [33], we accounted for head motion at the group level by including mean FD as a nuisance covariate [33, 34]. Group-level analyses were performed independently for each seed with SPM12. For each seed, all maps were entered into a general linear model with age, sex, FD, and baseline BMI as covariates, and the individual weight gain slope as the outcome measure. Significance was defined voxelwise at P < 0.001, with familywise error cluster correction at P < 0.05. We also included a region of interest analysis for brain areas implicated in obesity, including the dorsal anterior cingulate cortex (dACC; x = 3, y = −12, z = 36), right insula (rInsula; x = 38, y = 18, z = −3), right orbitofrontal cortex (rOFC; x = 34, y = 30, z = −3), and right superior frontal gyrus (rSFG; x = 22, y = 25, z = 51). For this analysis, we created spherical masks with a radius of 4 mm and extracted the mean beta estimates for each region of interest.
Finally, we combined the structural and functional indices of the striatum and their relationship with individual weight gain in an exploratory analysis. We therefore added functional connectivity indices, as well as the functional connectivity by volume interaction to an extended regression model, with individual weight gain slopes as the dependent measure. This model allowed us to test whether striatal volume moderated the association between fronto-striatal connectivity and weight gain. We then performed a leave-one-out cross-validation to derive a measure of predictive performance of this full model, and calculated the percentage of variance explained after cross-validation.
Results
Baseline differences
Patients and controls had similar weights and BMIs at baseline (that were also within the normal range; Table 1). There was no evidence that the duration of untreated psychosis correlated with BMI (β = −0.01, t (62) = −0.14, P = 0.89) or that prior exposure to antipsychotics (before entering the trial) was correlated with baseline BMI (β = 0.19, t (68) = 1.59, P = 0.117). Furthermore, non-drug-naive patients did not have a higher baseline BMI (M = 23.58, SD = 4.49) compared with healthy controls (M = 24.68, SD = 4.08). The left hippocampal volume was significantly reduced in patients compared with controls (Table 1), but no significant case–control differences were observed for any of the striatal regions.
Striatal volume and weight gain
We found that the average putamen volume was positively correlated with individual weight gain (β = 0.31, t (68) = 2.13, P = 0.036). Note that this finding did not depend on the selection of covariates we included in our model. To demonstrate this, we also tested for a correlation between putamen volume and individual weight gain, after adjusting for intracranial volume (β = 0.34, t (78) = 2.63, P = 0.01; Fig. S1a). Although it is good practice to adjust such an analysis for at least intracranial volume, the correlation (while difficult to interpret) is present even without this adjustment (β = 0.36, t (79) = 3.43, P < 0.001; Fig. S1b).
To explore the contributions of the left and right putamen separately, we then repeated this analysis with the left and right putamen volumes. The left putamen volume correlated with the magnitude of weight gain (Fig. 1a) in patients (β = 0.31, t (68) = 2.18, P = 0.033; Fig. 1b), with larger baseline volumes associated with greater weight gain during the subsequent trial.
Striatal volume correlates with weight gain in early-phase psychosis patients (N = 81). a Weight increased significantly over the course of the trial. Means with 95% confidence intervals are shown. b Weight gain in patients correlated with left putamen volume. Standardized beta coefficients with 95% confidence intervals are shown. All models were adjusted for age, sex, age by sex, duration of untreated psychosis, intracranial volume, and body mass index, none of which were significant predictors of weight gain. Error bars not touching the zero line indicate significant effects (P < 0.05). Caud, caudate; Hipp, hippocampus; Put, putamen; L, left, R, right
Striatal connectivity and weight gain
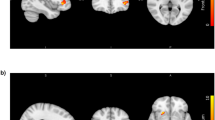
Following up on the left putamen volume finding, we tested for functional resting-state connectivity [27] between the left putamen and the whole brain that was associated with weight gain in 75 patients. Notably, we found similar patterns of functional connectivity (Fig. S2) as previous work in striatal connectivity [32], including our own work on a subsample of the current cohort [35]. For the left dorsal rostral putamen, we found that decreased functional connectivity with the lateral part of the right sensory motor cortex pole also correlated with the amount of weight gain (Fig. 2a, b). Note that the statistical significance of this result did not depend on the inclusion or omission of the covariates we chose. Left putamen volume and fronto-striatal connectivity were not significantly correlated (β = −0.05, t (73) = −0.39, P = 0.697).
Fronto-striatal connectivity and the interaction of striatal structure and function correlate with weight gain in early psychosis patients (N = 75). a, b Lower functional connectivity between the left putamen and the lateral part of the right motor cortex correlated with weight gain. Following up on the left putamen volume finding, we tested for functional connectivity between the left putamen and the whole brain that was associated with weight gain. The left dorsal rostral putamen (green) was used as seed region, and the lateral part of the right sensory motor cortex (red) was the only connected region surviving a voxel level threshold of P < 0.001 and a cluster threshold of P < 0.05 (corrected for familywise error). c Interaction of striatal structure and function correlated with weight gain in exploratory analysis. To illustrate the significant interaction between left putamen volume and connectivity, we plotted regression lines for weight gain and connectivity for the highest quartile (25%) of putamen volume and the lowest quartile (25%) of putamen volume
We did not find evidence that additional striatal connections with predefined regions that have been implicated in obesity (including anterior cingulate cortex, orbitofrontal cortex, and superior frontal gyrus) were associated with antipsychotic weight gain (Fig. S3).
Confirmation of results
Repeating the whole-brain analysis with global signal regressed out during preprocessing yielded essentially the same results (Fig. S4). Specifically, we found the same cluster in the right sensory motor cortex, where resting-state connectivity was negatively correlated with individual weight gain (Fig. S4b). Even though this connection did not survive cluster correction (P = 0.065, corrected for familywise error), it did survive correction for false discovery rate (P = 0.036).
Notably, the regression models were adjusted for age, sex, intracranial volume, baseline BMI, and duration of untreated psychosis; none of these variables showed a significant association with weight gain. In addition, to verify that the results were robust to the type of medication administered (risperidone vs. aripiprazole), we extended the models by including interactions of medication type and volume as well as medication type and connectivity, respectively, in the two regression models. There was no evidence for an effect of medication type on the weight gain and volume association (β = 0.01, t (66) = 0.11, P = 0.916) or the weight gain and connectivity association (β = 0.07, t (71) = 0.46, P = 0.648). In addition, no interactions with volume or connectivity were found for prior exposure to antipsychotics and medication dose during the trial.
Striatal structure and function interaction
These results suggest that patients with higher striatal volume and more negative fronto-striatal connectivity gained more weight during the clinical trial. In an exploratory fashion, we then investigated a potential synergistic effect of striatal structure and function. Therefore, we added functional connectivity indices as well as the functional connectivity by volume interaction to an extended regression model. This model allowed us to test whether striatal volume moderated the association between fronto-striatal connectivity and weight gain. Indeed, we found evidence for an interaction of volume and connectivity (β = −0.29, t (65) = −3.74, P < 0.001; Fig. 2c), indicating that the negative relationship between fronto-striatal connectivity and weight gain was weaker with lower compared with higher striatal volumes. Note that this effect remained significant after excluding patients who had bipolar I disorder and a recent manic episode with psychotic features (N = 3), and after adjusting the model for type of medication, medication dose during the trial, and prior exposure to antipsychotic drugs. We then assessed how well this full model predicted future weight gain in unseen data. Because we did not have an independent data set available, we addressed this question with leave-one-out cross-validation, and found that the model explained 29% of the variance after cross-validation.
Discussion
Here, we showed that striatal volume and fronto-striatal connectivity correlated with the degree of weight gain associated with antipsychotic treatment. Lower connectivity between the right sensory motor cortex and the left putamen was associated with more weight gain, and this relationship was stronger for higher compared with lower left putamen volume. In accordance with previous studies, we also found lower hippocampal volumes in patients compared with controls [36,37,38]. Yet, contrary to our expectation, we did not find that other brain areas implicated in obesity showed altered functional connectivity. In addition, we also did not find greater striatal volumes in patients compared with controls, possibly because our patients had only limited exposure to antipsychotic treatment.
We focused our study on the striatum, in line with this region’s important role in reward processing and weight gain. Previous studies have shown that treatment with antipsychotics in healthy controls induced an increase in reward activation in the dorsal striatum that was correlated with excessive eating [17] and that attenuated reward anticipation normalizes under treatment with antipsychotics in patients with schizophrenia [39, 40]. However, although altered reward processing is likely to play a role [16], antipsychotic-induced weight gain remains a complex issue [4]. First, one could ask why patients did not gain weight before the antipsychotic treatment if baseline alterations in reward processing are the main cause for weight gain. Indeed, there is evidence suggesting that patients might show metabolic aberrations already before they start treatment, possibly due to unhealthy lifestyle, illness neurobiology, and genetic factors [41,42,43,44]. Furthermore, it is important to consider that some patients in the current study were not naive to antipsychotic drugs but had been exposed to antipsychotic treatment prior to inclusion. Thus, weight gain may have occurred during this time frame. Nevertheless, we did not find evidence that baseline BMI correlated with days of prior drug exposure or that non-drug-naive patients had a higher baseline BMI compared with controls, speaking against substantial weight gain before the trial. The current trial of 12 weeks, although short, thus appears long enough to induce substantial weight gain with considerable inter-subject variability, especially in subjects with minimal prior antipsychotic exposure [1]. Second, one might ask how antipsychotics and some of their counterparts, dopaminergic agonists, can both induce weight gain, as shown for Parkinson disease [45]. A possible explanation is that weight gain in Parkinson disease is triggered mainly by compulsive eating [45, 46] whereas a decreased reward experience may be the main cause for weight gain induced by antipsychotics. It is also noteworthy that increased dopamine transmission through sympatomimetics, such as amphetamine, typically results in suppressed appetite.
One mechanism that likely contributes to weight gain is thus via altered reward processing in the striatum. The striatum is part of the brain’s reward circuitry, which has well-known dopaminergic components as well as neurotransmitters, including opioids, serotonin, and cannabinoids [9, 12, 17, 47, 48]. Accordingly, food consumption increased striatal dopamine in a previous study using positron emission tomography and this increase was positively correlated with meal pleasantness [12]. In addition, food cues elicited dorsal striatum dopamine increase and this increase correlated with hunger perception and food desire [49]. In obesity, dopamine has also been implicated in food reward and the control of eating behavior [50,51,52]. More specifically, functional magnetic resonance imaging studies in obesity have found altered activation in dopaminergic reward-related areas. Although these studies have described enhanced striatal activation in response to food-related cues [13, 53,54,55] the actual consumption of food elicited less activation in these areas [53, 54]. As previously suggested [17], these patterns are consistent with an imbalance between (increased) reward expectation and (decreased) reward experience, most likely through dopaminergic disruptions. Overeating could then be seen as a compensation to obtain the anticipated exaggerated reward [17].
Our study extends this model by considering the functional connectivity between the striatum and the sensory motor cortex, which has been implicated in food cue-related activity in obesity [13, 53, 54, 56, 57] Our study, however, found that the strength of this connectivity was correlated with less weight gain. Furthermore, we found that the association with weight gain was moderated by left putamen volume, with higher volumes together with lower connectivity correlating with more weight gain. The moderating role of the putamen volume suggests a synergistic effect of striatal structure and function, with increased putamen volumes, possibly due to reduced endogenous dopamine availability in the striatum [19, 20], multiplying the effect of lower cortico-striatal connectivity on weight gain [21, 22].
Some limitations merit comment. First, although the performance of the striatal structure and function model in predicting weight gain was promising (29% of the variance explained after cross validation), the sample size was small and we could not test the model performance in an independent data set. In addition, the functional connectivity measure that entered this model was not an independent measure but likely an overly optimistic estimate of the effect size of the fronto-striatal connectivity [58]. Nevertheless, if replicated in a larger sample, these data may help in the identification of patients at high risk for weight gain upon initiation of antipsychotic treatment. Furthermore, we used two different SGAs in this study, namely risperidone and aripiprazole. Although it is important to underscore that all current antipsychotics share affinity for the dopamine D2 receptors, they may still differ from a pharmacological point of view. For example, risperidone and aripiprazole share D2 receptor antagonism to induce an antipsychotic effect [59] but different receptor systems may be involved in side effects, such as weight gain. Apart from the striatum [16] weight gain has been associated with cortical 5-HT2A receptors in quetiapine monotherapy [60]. In addition, olanzapine exposure in healthy controls indicated negative effects on the peripheral metabolism. These findings suggest that antipsychotic-induced weight gain may involve additional central and peripheral aspects apart from the striatum. However, in the current study we did not find evidence that the type of medication interacted with the effects of striatal volume or fronto-striatal connectivity, suggesting similar effects of both drugs, which is also consistent with our previous finding for a larger trial [18] where no significant differences on weight gain were found between aripiprazole and risperidone. In addition, weight gain differences between aripiprazole and risperidone found in previous studies might reflect a difference in the sedative effect, with risperidone causing more sedation than aripiprazole [2]. Another limitation is that we used three putamen seeds, which we considered separately, thus not correcting for multiple comparisons across seeds. In addition, early-phase psychosis could have been defined differently, for instance, using the duration of untreated psychosis. However, consistent with our previous work, we used a criterion of cumulative exposure to antipsychotic treatment [18, 25, 26, 61]. Finally, future studies with repeated measurements of structural and functional imaging should investigate whether our findings reflect indeed a trait marker in patients prone to weight gain.
In conclusion, the current study showed that striatal structure and function correlated with antipsychotic-induced weight gain. Lower connectivity between the striatum and the sensory motor cortex was associated with more weight gain, and this relationship was stronger for higher compared with lower left putamen volumes in an exploratory analysis. This suggests that an imaging marker at baseline may identify patients who are prone to substantial weight gain during treatment.
Funding and disclosure
Presented at the 57th Annual Meeting of the American College of Neuropsychopharmacology (ACNP). Supported by NIMH grant P50MH080173 to Dr. Malhotra, grant R01MH060004 to Dr. Robinson, grant R01MH076995 to Dr. Szeszko and R21MH101746 to Drs. Robinson and Szeszko. Dr Robinson has been a consultant to Costello Medical Consulting, Innovative Science Solutions, Janssen, Lundbeck, Otsuka, and US WorldMeds and has received research support from Otsuka. Dr Malhotra has served as a consultant for Forum Pharmaceuticals and has served on a scientific advisory board for Genomind. Dr Lencz is a consultant for Genomind. The other authors report no financial relationships with commercial interests. The authors declare that they have no conflict of interest.
References
Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765.
Leucht S, Cipriani A, Spineli L, Mavridis D, Orrey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–62.
Musil R, Obermeier M, Russ P, Hamerle M. Weight gain and antipsychotics: a drug safety review. Exp Opin Drug Saf. 2015;14:73–96.
Kapur S, Marques T. Dopamine, striatum, antipsychotics, and questions about weight gain. JAMA Psychiatry. 2016;73:107–8.
Winkelbeiner S, Leucht S, Kane JM, Homan P. Evaluation of differences in individual treatment response in schizophrenia spectrum disorders: a meta-analysis. JAMA Psychiatry. 2019; Jun 3. doi: 10.1001/jamapsychiatry.2019.1530.
Malhotra AK, Correll CU, Chowdhury NI, Muller DJ, Gregersen PK, Lee AT, et al. Association between common variants near the melanocortin 4 receptor gene and severe antipsychotic drug-induced weight gain. Arch Gen psychiatry. 2012;69:904–12.
Brandl EJ, Tiwari AK, Zai CC, Nurmi EL, Chowdhury NI, Arenovich T, et al. Genome-wide association study on antipsychotic-induced weight gain in the catie sample. Pharm J. 2015;16:352–6.
Zhang J-P, Lencz T, Zhang RX, Nitta M, Maayan L, John M, et al. Pharmacogenetic associations of antipsychotic drug-related weight gain: a systematic review and meta-analysis. Schizophr Bull. 2016;42:1418–37.
Volkow ND, Wang G-J, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46.
Roerig JL, Steffen KJ, Mitchell JE. Atypical antipsychotic-induced weight gain. CNS Drugs. 2011;25:1035–59.
O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–4.
Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage. 2003;19:1709–15.
Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–21.
Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, et al. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS ONE. 2012;7:e31089.
Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30:13105–9.
Nielsen M, Rostrup E, Wulff S, Glenthoj B, Ebdrup BH. Striatal reward activity and antipsychotic-associated weight change in patients with schizophrenia undergoing initial treatment. JAMA Psychiatry. 2016;73:121–8.
Mathews J, Newcomer JW, Mathews JR, Fales CL, Pierce KJ, Akers BK, et al. Neural correlates of weight gain with olanzapine. Arch Gen Psychiatry. 2012;69:1226.
Robinson DG, Gallego JA, John M, Petrides G, Hassoun Y, Zhang J-P, et al. A randomized comparison of aripiprazole and risperidone for the acute treatment of first-episode schizophrenia and related disorders: 3-month outcomes. Schizophr Bull. 2015;41:1227–36.
Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry. 2001;158:486–9.
Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–24.
Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Haring H-U, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2012;33:1052–61.
Mole TB, Mak E, Chien Y, Voon V. Dissociated accumbens and hippocampal structural abnormalities across obesity and alcohol dependence. Int J Neuropsychopharmacol. 2016;19:pyw039.
Devoto F, Zapparoli L, Bonandrini R, Berlingeri M, Ferrulli A, Luzi L, et al. Hungry brains: a meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neurosci Biobehav Rev. 2018;94:271–85.
Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–23.
Homan P, Argyelan M, DeRosse P, Szeszko PR, Gallego JA, Hanna L, et al. Structural similarity networks predict clinical outcome in early-phase psychosis. Neuropsychopharmacology. 2019;44:915–22.
Robinson, DG, Gallego, JA, John, M, Hanna, LA, Zhang, J-P, Birnbaum, ML et al. A potential role for adjunctive omega-3 polyunsaturated fatty acids for depression and anxiety symptoms in recent onset psychosis: results from a 16-week randomized placebo-controlled trial for participants concurrently treated with risperidone. Schizophr Res. 2018;204:295–303.
Sarpal DK, Robinson DG, Fales C, Lencz T, Argyelan M, Karlsgodt KH, et al. Relationship between duration of untreated psychosis and intrinsic corticostriatal connectivity in patients with early phase schizophrenia. Neuropsychopharmacology. 2017;42:2214–21.
Hedeker D, Gibbons RD. Longitudinal data analysis. Volume 451. Hoboken, NJ: John Wiley & Sons; 2006.
Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage. 2017;154:174–87.
Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (compcor) for bold and perfusion based fMRI. NeuroImage. 2007;37:90–101.
Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH. Reduction of motion-related artifacts in resting state fMRI using acompcor. NeuroImage. 2014;96:22–35.
Di Martino A, Scheres A, Margulies DS, Kelly A, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: a resting state fMRI study. Cereb Cortex. 2008;18:2735–47.
Yan C-G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage. 2013;76:183–201.
Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fmri. NeuroImage. 2015;105:536–51.
Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72:5.
Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Cook M, et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia. Arch Gen Psychiatry. 1999;56:133.
Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. NeuroImage. 2004;21:1563–75.
Steen RG, Mull C, Mcclure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia. Br J Psychiatry. 2006;188:510–8.
Nielsen MO, Rostrup E, Wulff S, Bak N, Broberg BV, Lublin H, et al. Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch Gen Psychiatry. 2012;69:1195.
Nielsen MO, Rostrup E, Wulff S, Bak N, Lublin H, Kapur S, et al. Alterations of the brain reward system in antipsychotic naive schizophrenia patients. Biol Psychiatry. 2012;71:898–905.
Haupt DW, Newcomer JW. Abnormalities in glucose regulation associated with mental illness and treatment. J Psychosom Res. 2002;53:925–33.
Thakore JH, Mann JN, Vlahos I, Martin A, Reznek R. Increased visceral fat distribution in drug-naive and drug-free patients with schizophrenia. Int J Obes. 2002;26:137–41.
Kohen D. Diabetes mellitus and schizophrenia: historical perspective. Br J Psychiatry. 2004;184(S47):s64–66.
Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31:2091–120.
Nirenberg MJ, Waters C. Compulsive eating and weight gain related to dopamine agonist use. Mov Disord. 2006;21:524–9.
Vikdahl M, Carlsson M, Linder J, Forsgren L, Haglin L. Weight gain and increased central obesity in the early phase of parkinson’s disease. Clin Nutr. 2014;33:1132–9.
Cota D, Barrera JG, Seeley RJ. Leptin in energy balance and reward: two faces of the same coin? Neuron. 2006;51:678–80.
Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc B: Biol Sci. 2006;361:1149–58.
Volkow ND, Wang G-J, Fowler JS, Logan J, Jayne M, Franceschi D, et al. Nonhedonic food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–80.
Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–6.
Volkow ND, Wang G-J, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage. 2008;42:1537–43.
Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–60.
Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by taqia a1 allele. Science. 2008;322:449–52.
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–35.
Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–47.
Rapuano KM, Zieselman AL, Kelley WM, Sargent JD, Heatherton TF, Gilbert-Diamond D. Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proc Natl Acad Sci USA. 2016;114:160–65.
Contreras-Rodríguez O, Martín-Pérez C, Vilar-López R, Verdejo-Garcia A. Ventral and dorsal striatum networks in obesity: Link to food craving and weight gain. Biol Psychiatry. 2017;81:789–96.
Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–40.
Meltzer HY. New trends in the treatment of schizophrenia. CNS Neurol Disord—Drug Targets. 2017;16:900–6.
Rasmussen H, Ebdrup BH, Oranje B, Pinborg LH, Knudsen GM, Glenthoj B. Neocortical serotonin2a receptor binding predicts quetiapine associated weight gain in antipsychotic-naive first-episode schizophrenia patients. Int J Neuropsychopharmacol. 2014;17:1729–36.
Trampush JW, Lencz T, DeRosse P, John M, Gallego JA, Petrides G, et al. Relationship of cognition to clinical response in first-episode schizophrenia spectrum disorders. Schizophr Bull. 2015;41:1237–47.
Acknowledgements
The authors thank Dr Lauren Hanna and Dr Juan Gallego for their careful clinical oversight of the study. They acknowledge their patients, their patients’ families, and their psychiatry research support staff.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Homan, P., Argyelan, M., Fales, C.L. et al. Striatal volume and functional connectivity correlate with weight gain in early-phase psychosis. Neuropsychopharmacol. 44, 1948–1954 (2019). https://doi.org/10.1038/s41386-019-0464-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-019-0464-y
This article is cited by
-
Associations between antipsychotics-induced weight gain and brain networks of impulsivity
Translational Psychiatry (2024)
-
Interaction between baseline BMI and baseline disease severity predicts greater improvement in negative symptoms in first-episode schizophrenia
European Archives of Psychiatry and Clinical Neuroscience (2024)
-
Mechanism and treatments of antipsychotic-induced weight gain
International Journal of Obesity (2023)