Abstract
5-hydroxytryptophan (5-HTP) has shown therapeutic promise in a range of human CNS disorders. But native 5-HTP immediate release (IR) is poorly druggable, as rapid absorption causes rapid onset of adverse events, and rapid elimination causes fluctuating exposure. Recently, we reported that 5-HTP delivered as slow-release (SR) in mice augmented the brain pro-serotonergic effect of selective serotonin reuptake inhibitors (SSRIs), without the usual adverse events associated with 5-HTP IR. However, our previous study entailed translational limitations, in terms of route, dose, and duration. Here we modeled oral 5-HTP SR in mice by administering 5-HTP via the food. We modeled oral SSRI treatment via fluoxetine in the water, in a regimen recapitulating clinical pharmacokinetics and pharmacodynamics. 5-HTP SR produced plasma 5-HTP levels well within the range enhancing brain 5-HT function in humans. 5-HTP SR robustly increased brain 5-HT synthesis and levels. When administered with an SSRI, 5-HTP SR enhanced 5-HT-sensitive behaviors and neurotrophic mRNA expression. 5-HTP SR’s pro-serotonergic effects were stronger in mice with endogenous brain 5-HT deficiency. In a comprehensive screen, 5-HTP SR was devoid of overt toxicological effects. The present preclinical data, appreciated in the context of published 5-HTP clinical data, suggest that 5-HTP SR could represent a new therapeutic approach to the plethora of CNS disorders potentially treatable with a pro-serotonergic drug. 5-HTP SR might in particular be therapeutically relevant when brain 5-HT deficiency is pathogenic and as an adjunctive augmentation therapy to SSRI therapy.
Similar content being viewed by others
Introduction
5-hydroxytryptophan (5-HTP) is the endogenous rate-limiting precursor of 5-hydroxytryptamine (5-HT, serotonin) [1, 2]. In humans and animals, orally administered 5-HTP can reach the brain and enhance brain 5-HT function [3, 4]. Clinical pilot trials suggest that adjunctive (additive) 5-HTP augments the antidepressant efficacy of serotonin reuptake inhibitors [see referenes [5,6,7]]. There are also clinical reports that 5-HTP alleviates for example pain, obesity, and ataxia [8,9,10]. The human safety record of 5-HTP is good, with no reports of serious adverse events [reviewed in [2, 11]]. Overall, 5-HTP has shown therapeutic promise in a range of CNS disorders.
5-HTP has never been pursued as a therapeutic beyond the experimental stage. This is likely because fast pharmacokinetics makes 5-HTP impractical as a drug: 5-HTP’s short half-life, 2 h [12], entails intermittent pharmacological effect and 5-HTP’s rapid absorption entails maximal plasma level-related (CMax-related) gastrointestinal (GI) adverse events [13]. However, slow-release (SR) drug delivery can provide sustained drug exposure and lower CMax. In humans, SR delivery may markedly enhance efficacy and/or safety of fast pharmacokinetics compounds [14, 15]. Thus, it is conceivable that SR delivery could impart therapeutic properties on 5-HTP
Indeed, in mouse models, we recently reported that SR delivery markedly enhanced the therapeutic properties of 5-HTP [3]. SR delivery eliminated the typical GI and CNS adverse events associated with 5-HTP administration in rodents and, surprisingly, enabled higher safe 5-HTP plasma levels. Adjunctive 5-HTP SR potently augmented SSRI-induced 5-HTExt-elevation, recapitulating human 5-HTP findings [4]. While encouraging, this recent data set had translational limitations: 5-HTP SR was administered parenterally and sub-acutely and the SSRI dose was supra-clinical, saturating, and not selective for the serotonin transporter (SERT). In contrast, in humans a 5-HTP SR drug would be oral and administered chronically and therapeutic SSRI dosing is non-saturating and SERT-selective.
The present study expands on the former; but, employs methodological approaches better translating to a clinical scenario. The aim of the present study was to explore the pharmacokinetics, pharmacodynamics, safety, and thus druggability of oral 5-HTP SR. The aim was not to test oral 5-HTP SR in animal models of CNS disease. Substantial clinical evidence already suggests that an appropriate 5-HTP drug could treat various CNS diseases. Clinical efficacy evidence supersedes any animal disease model data—particularly for psychiatric disorders, where animal disease models are minimally predictive of human efficacy [16, 17]. The present study addressed the following questions in mouse models:
-
Can oral 5-HTP SR safely elevate plasma 5-HTP to pharmacologically active levels?
-
Can oral 5-HTP SR safely be combined with therapeutically-relevant SSRI treatment?
-
Can oral 5-HTP SR enhance brain 5-HT synthesis and levels?
-
Can oral 5-HTP SR, alone or adjunctive to an SSRI, enhance indicators of increased brain 5-HT function?
-
Will oral 5-HTP SR, alone or adjunctive to an SSRI, have differential effects under conditions of brain 5-HT deficiency?
Answering these questions in animals can inform whether an oral 5-HTP SR treatment would be feasible in humans.
Materials and methods
Animals
Mice were housed 3–5/cage with food and water available ad libitum on a 12 h light–dark cycle at 21 ± 1 °C. All experiments, except sexual behaviors, used female age-matched littermate wild type (WT) and tryptophan hydroxylase 2 Arg439His knockin mice (3–6-months-old). The latter mice, henceforth referred to as “5-HTHypo” mice, synthesize 20–30% of WT brain 5-HT levels [18, 19]. We used the 5-HTHypo mice to model naturalistic brain 5-HT deficiency. Experiments were conducted in accordance with the National Institutes of Health guidelines for the care and use of animals and an approved protocol from the Duke University Animal Care and Use Committee.
Materials
Fluoxetine was from Sequoia Research Products (Pangbourne, UK). 5-HTP was from EMD Millipore (Billerica, MA). 5-HT, 5-HIAA, and dopamine were from Sigma (St Louis, MO). Flesinoxan was a gift from Lundbeck (Valby, DK). All chemicals used were of analytical grade.
SSRI treatment
Chronic SSRI treatment was modeled by fluoxetine in the drinking water, 25 mg/L, corresponding to ~4 mg/kg/day (assuming a water intake of 150 mL/day). Pilot studies found chronic fluoxetine 25 mg/L to produce fluoxetinePlasma levels of ~100 ng/ml, equivalent to average clinical levels (Fig. 1a). Fluoxetine water in darkened bottles was changed twice weekly. FluoxetinePlasma was determined using HPLC-MS as described [20]. SERT occupancy after fluoxetine treatment was determined ex vivo on brain slices as described [3]. 5-HT1A receptor agonist hypothermia was assessed essentially as described [18], but using flesinoxan (3.3 mg/kg, I.P., in saline/10% DMSO) as the 5-HT1A receptor agonist. In brief, three baseline and eight post-injection core body temperature readings were recorded in 15 min intervals. Temperature change was expressed as change from the average of the three baseline measurements.
Clinically relevant fluoxetine and oral 5-HTP SR treatments. a Average plasma fluoxetine and nor-fluoxetine levels (measured at ZT0) after 2 weeks of oral fluoxetine (4 mg/kg/day) are similar to clinical averages. Dotted lines = clinical averages; shaded areas = clinical standard deviation [reported in [25]] (WT, n = 11; 5-HTHypo, n = 10). b Clinically relevant SERT occupancy after fluoxetine treatment (WT, n = 8; 5-HTHypo, n = 8). c, d As in humans, fluoxetine blunts the hypothermic response to a 5-HT1A agonist (flesinoxan, 3.3 mg/kg, I.P.) (WT, n = 6–8; 5-HTHypo, n = 8–9). e Treatment groups. f Average 5-HTPPlasma levels over 24 h (WT, n = 8–10; 5-HTHypo, n = 6–9). g Treatment and timeline schematic. Statistics: (a, b) t-test. (c, d) Two-way RM-ANOVA, followed by Bonferroni post hoc test. f Two-way ANOVA, followed by Bonferroni post-hoc test. *p < 0.05
5-HTP treatments
Oral 5-HTP SR was modeled by dietary 5-HTP, 6.7 mg/g chow, equivalent to ~1000 mg/kg/day [21], and was commenced following 2 weeks of fluoxetine/control treatment (Fig. 1g). This was the highest 5-HTP dose the mice would eat (not shown). Control mice received standard chow (See Supplementary Methods for details). Adjunctive oral 5-HTP immediate release (IR) was modeled by oral gavage, 100 mg/kg (in saline, 10 ml/kg), following 2 weeks of fluoxetine treatment. Gavage with saline served as control.
Tissue and plasma sample collection
All mice were sacrificed at Zeitgeber time 0 (ZT0) by cervical decapitation. Heads were instantly cooled to 0 °C. Whole brains were extracted, frozen on dry ice, and stored at −80 °C. Later, at −20 °C, the frontal cortex (orbit anterior to corpus callosum) was dissected, and from 1 mm thick brain slabs were punched (1.5 mm punches) the hippocampus (2 punches) and the nucleus accumbens (1 punch). One centimeter of jejunum was collected 1 cm below the distal duodenum. Plasma was prepared from trunk blood within 60 min of collection. For diurnal plasma 5-HTPPlasma assessment during oral 5-HTP SR, 15 µl tail-vein blood was collected as described [3] at ZT0, ZT6, ZT12, and ZT18. For 5-HTPPlasma assessment following oral 5-HTP IR, 15 µl tail-vein blood was collected, at 0, 15, 30, 45, and 60 min post-gavage. All samples were stored at −80 °C.
5-HTExt sensitive behaviors
All tests were performed between ZT1 and ZT6. Marble burying was performed as described [18]. In novelty suppressed feeding (NSF) the mice were presented for 5 days with one peanut butter chip (Reese’s, Hershey, PA) in a 2″ × 2″ plastic dish, one chip per mouse in a cage. On day 6, one chip in a dish was presented in a white, novel arena (20″ × 30″). Latency to eat was scored blind from videos, cut-off 600 s.
Physiological toxicology
Diarrhea was scored blindly on a scale from 0 (none) to 3 (severe), as described [3]. For adjunctive oral 5-HTP SR, diarrhea was scored after 2 days and 7 days at ZT1. For 5-HTP IR, diarrhea was scored 0–30 min after gavage. Head-twitches were counted from videos by a blinded observer. For adjunctive oral 5-HTP SR, head-twitches were counted over 5 min after 2 and 7 days at ZT1. For 5-HTP IR, head-twitches were counted 0–30 min after gavage. Body weights were recorded weekly.
Behavioral toxicology
All tests were performed between ZT1 and ZT6. Locomotor activity (total distance, rearing, and center time) was recorded over 30 min in 40 × 40 cm photocell activity monitors, as previously described [3]. Y-maze spontaneous alternations, a measure of spatial working memory, were scored over 5 min as described [22]. Arm dimensions were 35 (l) × 10 (w) × 25 (h) cm. Social interaction was assessed by pairing with a novel female mouse in a neutral environment (clean cage). Social investigation (allo-sniffing) and aggression (fighting, stand-offs, tail rattling) were scored blinded over 5 min from videos. Beam walking, a measure of motor strength and coordination, was assessed over 2 min, as described [23]. Male sexual behaviors were assessed over 15 min after introduction of an estrous female into the home cage of a single-housed male. Mounting number and duration, sexual interest (ano-genital sniffing), and social investigation (allo-sniffing) were scored blinded from videos.
5-HTP absolute bioavailability
5-HTP was administered orally (60 mg/kg) or intravenously (20 mg/kg) and tail blood samples for 5-HTP quantitation were collected at 5, 15, 30, 60, 90, 120, 180, and 240 min. 5-HTPPlasma area under the curve (AUC) was computed. Absolute bioavailability (F): \(\frac{{AUC_{\mathrm{Oral}}}}{{AUC_{\mathrm{IV}}}} \times \frac{{Dose_{\mathrm{IV}}}}{{Dose_{\mathrm{Oral}}}}\)
Test sequences
Mice were tested in a pre-specified sequence before final euthanasia and tissue collection (Table S2).
Monoamine analysis
5-HTTissue, 5-HIAATissue, 5-HTPTissue, and 5-HTPPlasma were quantified by high-performance liquid chromatography (HPLC) with electrochemical detection as described [3, 18].
mRNA quantitation by real-time PCR
mRNA levels were determined as described [24]. In brief, RNA was extracted, reverse transcribed to cDNA, and cDNA levels quantified by real-time PCR. Primers: Glyceraldehydes 3-phosphate dehydrogenase (GAPDH): 5′-CAA CTT TGG CAT TGT GGA AGG-3′ and 5′-GTG GAT GCA GGG ATG ATG TT-3′. Brain-derived neurotrophic factor (BDNF): 5′-CAA TGC CGA ACT ACC CAA-3′ and 5′-AAC ATA AAT CCA CTA TCT TCC CC-3′. Fibroblast growth-factor 2 (FGF-2): 5′-CCT TGC TAT GAA GGA AGA TGG A-3′ and 5′- CCG TGA CCG GTA AGT ATT GTA G-3′.
Statistical analysis
Data were analyzed with unpaired Student’s t-test, one-way analysis of variance (ANOVA), two-way ANOVA, or two-way repeated measures (RM)-ANOVA, as appropriate, using Prism statistical software version 5.0 f (GraphPad Software, La Jolla, CA, USA). When significant factor effects or factor interactions were detected, analysis of variance was followed by Bonferroni’s post-hoc test, or, when comparison to the untreated Ctrl + Ctrl group was most relevant, Dunnett’s post hoc test. Our focus was the effect of adjunctive oral 5-HTP SR, not tph2 genotype. Therefore, WT and 5-HTHypo data were analyzed separately. Data are presented as mean ± standard error of mean (SEM). Detailed statistical data including F-values and degrees of freedom are presented in Table S1.
Results
The SSRI paradigm recapitulates a clinical scenario
To evaluate the translational fidelity of our SSRI paradigm, we determined if fluoxetinePlasma levels, SERT occupancy, and 5-HT1A agonist hypothermia corresponded to a therapeutic scenario. In both WT and 5-HTHypo mice, fluoxetinePlasma and nor-fluoxetinePlasma levels were within or close to one standard deviation of mean therapeutic levels (Fig. 1a) [25]. SERT occupancy was above 80 %, but did not reach saturation (Fig. 1b), as observed in SSRI-treated patients [26]. As previously reported [18], 5-HT1A agonist hypothermia was reduced in 5-HTHypo compared to WT mice (Fig. 1c, d). As in humans [27], fluoxetine blunted the hypothermic response. The blunting was less in 5-HTHypo mice, probably reflecting that chronic fluoxetine elevates 5-HTExt only minimally in 5-HTHypo mice [3, 24].
Oral 5-HTP SR elevates 5-HTPPlasma
During oral 5-HTP SR treatment, the average 24-h 5-HTPPlasma levels were ~3000 ng/ml (Fig. 1f). There were no significant treatment differences between groups of mice treated with oral 5-HTP SR.
Oral 5-HTP SR markedly elevates brain 5-HT synthesis and tissue levels
Oral 5-HTP SR elevated 5-HTTissue in the frontal cortex, hippocampus, and nucleus accumbens, irrespective of fluoxetine co-treatment. In 5-HTHypo mice, oral 5-HTP SR restored 5-HTTissue to WT levels in all brain regions (Fig. 2a, d, g). Oral 5-HTP SR also elevated 5-HIAATissue several-fold in all regions, in both genotypes (Fig. 2b, e, h). Likewise, 5-HTPTissue was elevated many-fold (Fig. 2c, f). Lastly, in the nucleus accumbens we also quantified DATissue, and found no treatment effects (Fig. 2i).
Oral 5-HTP SR elevates brain 5-HT synthesis, tissue levels and metabolism, and does not affect DA levels. a–c Frontal cortex 5-HTTissue, 5-HIAATissue, and 5-HTPTissue levels (WT, n = 8–10; 5-HTHypo, n = 7–9). d–f Hippocampal 5-HTTissue, 5-HIAATissue, and 5-HTPTissue levels (WT, n = 11–12; 5-HTHypo, n = 9–13). g–i Nucleus accumbens 5-HTTissue, 5-HIAATissue, and DATissue levels (WT, n = 6–10; 5-HTHypo, n = 7–9). Statistics: two-way ANOVA, followed by Bonferroni post hoc test. *p < 0.05
Adjunctive oral 5-HTP SR causes no adverse effects
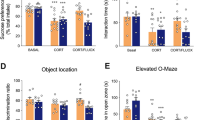
We assessed the effects of oral 5-HTP SR on safety measures related to physiology, motor activity, motor coordination, working memory, social behaviors, and sexual behaviors (Fig. 3). Oral 5-HTP SR—alone or adjunctive to fluoxetine—did not cause diarrhea or mild seizures (head-twitches), and did not affect body weight gain, spontaneous activity, motor coordination (beam walking), spatial working memory (Y-maze alternations), social interest (allo-sniffing), or sexual interest (male ano-genital sniffing of an estrous female). We also scored mounting sexual behavior and observed no apparent effects of oral 5-HTP SR, although low levels of mounting limited this assessment (Fig. S1). In WT mice only, fluoxetine modestly decreases forward locomotion (Fig. 3e, k) and, surprisingly, enhanced spatial working memory (Fig. 3m).
Adjunctive oral 5-HTP SR causes no physiological or behavioral adverse effects. a Diarrhea and b head-twitch (mild seizures) quantitation at day 2 and 7 after commencement of oral 5-HTP SR treatment (WT, n = 11–15; 5-HTHypo, n = 10–13). c, d) Body weight gain from 14 days prior to 28 days after commencement of oral 5-HTP SR treatment (WT, n = 11–15; 5-HTHypo, n = 10–13). Spontaneous activity e, f total distance, g, h vertical activity, and i, j center time (WT, n = 11–15; 5-HTHypo, n = 10–13). Beam walking k zone crossings and l latency to fall (cut-off 120 s) (WT, n = 11–15; 5-HTHypo, n = 10–13). Y-maze m spontaneous alternations (50 % = chance) and n arm entries (activity control parameter) (WT, n = 15–21; 5-HTHypo, n = 14–21). o Social interaction with a novel same-sex mouse (WT, n = 11–14; 5-HTHypo, n = 9–13). p Male sexual interest for an estrous female introduced into the home cage (WT, n = 9–13; 5-HTHypo, n = 9–11). Statistics: two-way ANOVA, followed by Bonferroni post hoc test. *p < 0.05
Oral adjunctive 5-HTP IR causes significant adverse effects
Adjunctive oral 5-HTP IR 100 mg/kg caused strong diarrhea in both WT and 5-HTHypo mice, and mild seizures (head-twitches) in 5-HTHypo mice (Fig. 4b, c). Adjunctive 5-HTP IR transiently elevated 5-HTPPlasma to 4000–6000 ng/ml (Fig. 4d, e).
A one-tenth dose of adjunctive oral 5-HTP IR, as compared to oral 5-HTP SR, causes adverse effects. a Treatment timeline schematic. b Diarrhea, and c head-twitch (mild seizures) quantitation (WT, n = 8–9; 5-HTHypo, n = 8–9). d, e 5-HTPPlasma levels (5-HTP IR 100 mg/kg PO at T = 0 min) (WT, n = 8; 5-HTHypo, n = 7). Statistics: b, c t-test. d, e Two-way RM-ANOVA, followed by Bonferroni post hoc test. *p < 0.05
Adjunctive oral 5-HTP SR augments the effects of SSRI treatment in 5-HT-sensitive behaviors
We tested the behavioral effects of oral 5-HTP SR in several paradigms commonly responsive to enhanced brain 5-HT function. In novelty suppressed feeding, in both WT and 5-HTHypo mice, fluoxetine treatment alone had no effect. Oral 5-HTP SR decreased latency to feed, and there was a statistically significant interaction between fluoxetine and oral 5-HTP SR. We performed post hoc analysis to compare the three treatment groups individually to untreated controls. In WT mice, fluoxetine + oral 5-HTP SR treatment significantly decreased feeding latency compared to untreated control. In 5-HTHypo mice, both oral 5-HTP SR alone and fluoxetine + oral 5-HTP SR significantly decreased feeding latency compared to untreated control (Fig. 5a). In marble burying, in both WT and 5-HTHypo mice, both fluoxetine and oral 5-HTP SR inhibited marble burying behavior. In WT mice, there was a statistically significant interaction between fluoxetine and oral 5-HTP SR. In both WT and 5-HTHypo mice, post hoc analysis found that only the fluoxetine + oral 5-HTP SR treatment groups significantly inhibited marble burying, as compared to untreated controls (Fig. 5b). In the forced swim test, the only effect was that fluoxetine increased immobility (Fig. S2A), a not uncommon finding [28]. In the tail suspension test, we observed no treatments effects (Fig. S2B), again, for fluoxetine, not an uncommon finding [29].
Adjunctive oral 5-HTP SR augments 5-HT-sensitive behaviors and induces growth factor mRNA transcription. a Novelty suppressed feeding, latency to eat a peanut chip (WT, n = 17–24; 5-HTHypo, n = 17–21). b Marble burying, marbles buried in 15 min (WT, n = 9–12; 5-HTHypo, n = 9–11). c BDNF and d FGF2 mRNA in the hippocampus (WT, n = 7–10; 5-HTHypo, n = 6–8). e BDNF and f FGF2 mRNA in the nucleus accumbens (WT, n = 7–10; 5-HTHypo, n = 6–8). Statistics: two-way ANOVA, followed by Dunnett’s post hoc test. Brackets indicate significant difference from Ctrl + Ctrl group. *p < 0.05
Under 5-HT deficiency, adjunctive oral 5-HTP SR augments SSRI-induced gene transcription of neurotrophic factors
In WT mice, we found that fluoxetine decreased hippocampal BDNF and FGF2 mRNA levels (Fig. 5c, d). In contrast, in 5-HTHypo mice both fluoxetine and oral 5-HTP SR elevated hippocampal BDNF and FGF2 mRNA levels. Post-hoc testing found that only the fluoxetine + oral 5-HTP SR treatment group significantly elevated BDNF and FGF2 mRNA levels in 5-HTHypo mice as compared to untreated controls (Fig. 5c, d). In the nucleus accumbens there were no treatments effects (Fig. 5e, f).
5-HTP bioavailability
Oral administration of 5-HTP 60 mg/kg produced a slightly larger 5-HTPPlasma AUC compared to IV administration of 5-HTP 20 mg/kg (Fig. S3A). Adjusting for dose, absolute bioavailability was computed to F = 40% (Fig. S3B).
Oral 5-HTP SR has minimal effects on jejunal 5-HTTissue levels
In WT mice fluoxetine modestly decreased while oral 5-HTP SR modestly increased 5-HTTissue in the jejunum. In 5-HTHypo mice a similar trend was apparent, but it did not reach statistical significance. In both genotypes, levels of 5-HIAATissue were many-fold increased, reflecting that a substantial fraction of oral 5-HTP SR is metabolized via the 5-HT pathway in the intestine (Fig. S4).
Discussion
5-HTP has shown therapeutic promise in mood and other CNS disorders. But the pharmacokinetics of native 5-HTP (IR) is too rapid for general drug therapy. Here, using translationally optimized mouse paradigms, we tested the hypothesis that SR delivery would substantially improve the therapeutic properties of 5-HTP.
We report that oral 5-HTP SR can safely elevate plasma 5-HTP to pharmacologically active levels, can enhance brain 5-HT synthesis and levels, can safely be combined with SSRI treatment, and can augment the SSRI pharmacological effect. Additionally, oral 5-HTP SR has more pronounced pro-serotonergic effects under conditions of brain 5-HT deficiency.
The present study has advantages and limitations. Advantages include chronic oral administration, clinically relevant SSRI exposure, assessment of SSRI and 5-HTP SR effects under normal and 5-HT-deficient conditions, and a comprehensive multi-domain screen for adverse effects. One limitation is the inherent difficulty of emulating oral SR delivery in mice. Dietary administration provides a reasonable approach, as drug delivery is distributed throughout the 24-h day. Another limitation is that brain 5-HTExt could not be quantified, i.e. with microdialysis. The required invasive procedures would alter dietary consumption, which would alter drug delivery, rendering 5-HTExt data uninterpretable in the context of the main body of data presented. Further, for this first oral proof-of-concept study, due to resource limitations, we studied only one oral 5-HTP SR dose and used female mice only in most paradigms. However, such limitations do not impact the overall ramifications, that SR delivery markedly improves the drug properties of oral 5-HTP.
Oral 5-HTP SR produced average steady state 5-HTPPlasma levels of ~3000 ng/ml. In humans, after 5-HTP administration, reported 5-HTPPlasma levels sufficient to enhance indices of brain 5-HT function ranges from several hundreds to low thousands ng/ml [4, 12, 30]. Thus, the 5-HTPPlasma levels observed after oral 5-HTP SR are consistent with enhancement of brain 5-HT function. After oral 5-HTP IR, CMax occurred at the first time-point, 15 min, indicating rapid absorption of 5-HTP from the intestine, as is also observed in humans [12].
In drug discovery, good safety is equally important to therapy-like pharmacokinetics and pharmacodynamics. In rodents, 5-HTP IR, in medium to high parenteral bolus doses (10–200 mg/kg), can induce mild seizures (head-twitch), diarrhea, and motor anomalies in mice, toxic effects exacerbated by SSRI co-treatment [31,32,33]. Here we report that oral 5-HTP SR, in a dose of 1000 mg/kg/day, alone or as adjunctive to SSRI treatment, causes no discernable adverse events in a comprehensive toxicology screen assessing physiological, motoric, behavioral, and cognitive parameters. Importantly, we detected no signs of serotonin syndrome. In contrast, adjunctive oral 5-HTP IR—at 100 mg/kg, a tenth of the dose of 5-HTP SR—caused diarrhea in both genotypes and head-twitches in 5-HTHypo mice. This stark difference in safety between 5-HTP SR and IR parallels our previous finding investigating the pre-clinical pharmacology and safety of parenteral 5-HTP SR and IR [3]. Interestingly, rapid onset diarrhea and other GI events are among the most common and significant safety findings with oral 5-HTP IR in human trials [34]. Likely, the rapid onset adverse effects of 5-HTP IR can be accounted for by a rapid spike in 5-HTPPlasma and a high CMax [35]. Additionally, it is conceivable that the two weeks pre-treatment with SSRI prior to adding oral 5-HTP SR contributed to the absence of overt adverse events with the combined treatment, by ensuring that 5-HT function was enhanced step-wise.
We assessed the pharmacodynamic effects of oral 5-HTP SR, alone or adjunctive to SSRI treatment, on the monoamine, behavioral, and gene transcription levels. In both genotypes, oral 5-HTP SR produced a large elevation in brain 5-HTP, demonstrating that a substantial proportion of orally administered 5-HTP reaches the brain. In WT mice, oral 5-HTP SR only moderately elevated 5-HTTissue, i.e. 5-HT neuronal storage. However, this may not represent the magnitude of enhancement of brain 5-HT function, as our previous report found that parenteral 5-HTP SR in WT mice doubled neuronal 5-HT release (i.e. 5-HTExt) despite not altering brain 5-HTTissue levels. An explanation could be that 5-HT neuronal storage in WT mice at baseline is already close to capacity. Consistent with this notion, in 5-HTHypo mice, where 5-HT neuronal storage is reduced by 80% [19], oral 5-HTP SR strongly elevated 5-HTTissue, restoring levels to WT levels. In both genotypes, 5-HIAATissue was many-fold elevated. This indicates greatly enhanced flow through the 5-HT metabolic pathway, which is generally associated with increased 5-HT function. As previously reported with parenteral 5-HTP SR [3], DATissue was unaffected by oral 5-HTP SR. Thus, oral 5-HTP SR caused a marked enhancement of brain 5-HT synthesis, levels, and metabolism, and seemingly without interfering with storage of other monoamines.
Only the combined treatment with SSRI and oral 5-HTP SR reliably enhanced 5-HT-sensitive behaviors and hippocampal neurotropic gene transcription. These findings could reflect human and animal findings, ours and others, that SSRIs and 5-HTP synergize in enhancing 5-HT function [3, 4, 36]. In contrast to in the hippocampus, no treatment affected nucleus accumbens neurotrophic gene transcription. The reason for this region-dependency is not clear. However, it is note-worthy that nucleus accumbens BDNF appears to a mediator of stress responses rather than of antidepressant effects [37].
As explained above, methodological constraints inherently precluded directly quantifying brain 5-HTExt. However, it is justified to surmise that adjunctive oral 5-HTP SR substantially elevated brain 5-HTExt. First, previously we reported that 5-HTPPlasma levels of ~1000 ng/ml, after parenteral 5-HTP SR 100 mg/kg/day, corresponded to one- to several-fold elevations in brain 5-HTExt, whether above baseline or above the effect caused by SSRI treatment [3]. In the present study, oral 5-HTP SR 1000 mg/kg/day produced 5-HTPPlasma levels of ~3000 ng/ml, three times higher than parenteral 5-HTP SR. Second, oral 5-HTP SR produced substantially more robust elevation of brain 5-HTTissue than did parenteral 5-HTP SR. Third, adjunctive oral 5-HTP SR impacted 5-HT-sensitive behaviors and gene transcription, indicating functionally active 5-HTExt elevation.
The long half-life of fluoxetine and the active metabolite in rodents makes fluoxetine ideal for modeling SSRI therapy [38], as in human therapy SSRI exposure fluctuates minimally. We observed fluoxetinePlasma levels, SERT occupancy, and a blunting of 5-HT1AR agonist hypothermia corresponding to that observed during human SSRI therapy [25, 27, 39]. Thus, our SSRI regimen recapitulated a clinical scenario, which is critical for a translational study.
Similar to other reports using therapeutically relevant SSRI regimens, we observed minimal effects of SSRI alone on 5-HT-sensitive behaviors and gene transcription [40, 41]. These minimal pharmacodynamic effects may reflect human and rodent data that clinically relevant SSRI regimens elevate 5-HTExt only moderately [42, 43]. In mice, higher fluoxetine doses, 10–20 mg/kg/day, typically have more pronounced pharmacodynamic, e.g. behavioral, neurotrophic, effects [24, 44]. However, these higher fluoxetine doses will produce fluoxetinePlasma levels over ten-fold higher than therapeutic levels [20, 45]. At such supra-clinical levels fluoxetine significantly engages multiple transporters, receptors, and ion channels, not engaged during a therapeutic scenario [46]. Such off-target effects would have confounded the present study, which aimed to focus on 5-HT function and aimed to maximize translational fidelity.
Conclusion
The present report demonstrates, in animal models, that SR delivery markedly and to an unexpected degree improves the therapeutic properties of oral 5-HTP. The present data should be appreciated in the context of the large body of clinical data suggesting that 5-HTP has therapeutic potential but pharmacokinetics incompatible with general therapy, and appreciated in the context of our previous report on parenteral 5-HTP SR [3]. A 5-HTP SR drug could be particularly relevant as an adjunctive to augment SSRI therapy, e.g. depression refractory to SSRI therapy [2], and in conditions associated with 5-HT deficiency, e.g. impulse control disorders and suicidality [47]. CNS drug concepts can take decades to mature from initial clinical findings to established therapy [48, 49]. In the case of 5-HTP, only recently has drug delivery technologies become available to enable a 5-HTP SR drug [35]. Altogether, clinical and preclinical data suggest that a 5-HTP SR drug could represent a new treatment option for mood and other CNS disorders treatable with serotonergic drugs.
Funding and disclosure
This work was supported in part by grants from the National Institutes of Health MH79201 and MH60451 (MGC). BDS was the recipient of a Minority Supplement award from the National Institutes of Health (MH79201–03S1). JPRJ was the recipient of an individual grant from The Lundbeck Foundation of Denmark. Support from the Lennon Family Foundation to MGC for earlier foundational work is greatly appreciated. MGC and JPRJ are inventors on issued and pending patents in relation to the therapeutic use of 5-HTP SR. MGC and JPRJ are shareholders in Evecxia Inc., a company founded to develop and commercialize 5-HTP SR therapeutics. The remaining authors have nothing to disclose.
References
Davidson J, Sjoerdsma A, Loomis LN, Udenfriend S. Studies with the serotonin precursor, 5-hydroxytryptophan, in experimental animals and man. J Clin Invest. 1957;36:1594–9.
Jacobsen JP, Krystal AD, Krishnan KR, Caron MG. Adjunctive 5-Hydroxytryptophan slow-release for treatment-resistant depression: clinical and preclinical rationale. Trends Pharmacol Sci. 2016a;37:933–44.
Jacobsen JP, Rudder ML, Roberts W, Royer EL, Robinson TJ, Oh A, et al. SSRI augmentation by 5-hydroxytryptophan slow release: mouse pharmacodynamic proof of concept. Neuropsychopharmacology. 2016b;41:2324–34.
Sargent PA, Williamson DJ, Cowen PJ. Brain 5-HT neurotransmission during paroxetine treatment. Br J Psychiatry: J Ment Sci. 1998;172:49–52.
Nardini M, De Stefano R, Iannuccelli M, Borghesi R, Battistini N. Treatment of depression with L-5-hydroxytryptophan combined with chlorimipramine, a double-blind study. Int J Clin Pharm Res. 1983;3:239–50.
van Hiele LJ. l-5-Hydroxytryptophan in depression: the first substitution therapy in psychiatry? The treatment of 99 out-patients with ‘therapy-resistant’ depressions. Neuropsychobiology. 1980;6:230–40.
van Praag HM. Serotonin precursors in the treatment of depression. Adv Biochem Psychopharmacol. 1982;34:259–86.
Trouillas P, Brudon F, Adeleine P. Improvement of cerebellar ataxia with levorotatory form of 5-hydroxytryptophan. A double-blind study with quantified data processing. Arch Neurol. 1988;45:1217–22.
Cangiano C, Ceci F, Cascino A, Del Ben M, Laviano A, Muscaritoli M, et al. Eating behavior and adherence to dietary prescriptions in obese adult subjects treated with 5-hydroxytryptophan. Am J Clin Nutr. 1992;56:863–7.
Caruso I, Sarzi Puttini P, Cazzola M, Azzolini V. Double-blind study of 5-hydroxytryptophan versus placebo in the treatment of primary fibromyalgia syndrome. J Int Med Res. 1990;18:201–9.
Das YT, Bagchi M, Bagchi D, Preuss HG. Safety of 5-hydroxy-L-tryptophan. Toxicol Lett. 2004;150:111–22.
Gijsman HJ, van Gerven JM, de Kam ML, Schoemaker RC, Pieters MS, Weemaes M, et al. Placebo-controlled comparison of three dose-regimens of 5-hydroxytryptophan challenge test in healthy volunteers. J Clin Psychopharmacol. 2002;22:183–9.
Lowe SL, Yeo KP, Teng L, Soon DK, Pan A, Wise SD, et al. L-5-Hydroxytryptophan augments the neuroendocrine response to a SSRI. Psychoneuroendocrinology. 2006;31:473–84.
Croom KF, Wellington K. Modified-release nifedipine: a review of the use of modified-release formulations in the treatment of hypertension and angina pectoris. Drugs. 2006;66:497–528.
Zhang Y, Britto MR, Valderhaug KL, Wedlund PJ, Smith RA. Dextromethorphan: enhancing its systemic availability by way of low-dose quinidine-mediated inhibition of cytochrome P4502D6. Clin Pharmacol Ther. 1992;51:647–55.
Hyman SE. Revolution stalled. Sci Transl Med. 2012;4:155cm111.
Insel TR. Next-generation treatments for mental disorders. Sci Transl Med. 2012;4:155ps119.
Jacobsen JP, Siesser WB, Sachs BD, Peterson S, Cools MJ, Setola V, et al. Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice. Mol psychiatry. 2012b;17:694–704.
Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008;105:1333–8.
Siesser WB, Sachs BD, Ramsey AJ, Sotnikova TD, Beaulieu JM, Zhang X, et al. Chronic SSRI Treatment Exacerbates Serotonin Deficiency in Humanized Tph2 Mutant Mice. ACS Chem Neurosci. 2013;4:84–88.
Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–43.
Jacobsen JP, Redrobe JP, Hansen HH, Petersen S, Bond CT, Adelman JP, et al. Selective cognitive deficits and reduced hippocampal brain-derived neurotrophic factor mRNA expression in small-conductance calcium-activated K+channel deficient mice. Neuroscience. 2009;163:73–81.
Jacobsen JP, Weikop P, Hansen HH, Mikkelsen JD, Redrobe JP, Holst D, et al. SK3 K+channel-deficient mice have enhanced dopamine and serotonin release and altered emotional behaviors. Genes Brain Behav. 2008;7:836–48.
Sachs BD, Jacobsen JP, Thomas TL, Siesser WB, Roberts WL, Caron MG. The effects of congenital brain serotonin deficiency on responses to chronic fluoxetine. Transl Psychiatry. 2013;3:e291.
Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Michelson D, et al. Fluoxetine and norfluoxetine plasma concentrations in major depression: a multicenter study. Am J psychiatry. 1997;154:963–9.
Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J psychiatry. 2004;161:826–35.
Lerer B, Gelfin Y, Gorfine M, Allolio B, Lesch KP, Newman ME. 5-HT1A receptor function in normal subjects on clinical doses of fluoxetine: blunted temperature and hormone responses to ipsapirone challenge. Neuropsychopharmacology. 1999;20:628–39.
Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology. 2001;155:315–22.
Benton CS, Miller BH, Skwerer S, Suzuki O, Schultz LE, Cameron MD, et al. Evaluating genetic markers and neurobiochemical analytes for fluoxetine response using a panel of mouse inbred strains. Psychopharmacology. 2012;221:297–315.
Magnussen I, Van Woert MH. Human pharmacokinetics of long term 5-hydroxytryptophan combined with decarboxylase inhibitors. Eur J Clin Pharm. 1982;23:81–6.
Pascual D, Alsasua A, Goicoechea C, Martin MI. The involvement of 5-HT3 and 5-HT4 receptors in two models of gastrointestinal transit in mice. Neurosci Lett. 2002;326:163–6.
Sanchez C, Kreilgaard M. R-citalopram inhibits functional and 5-HTP-evoked behavioural responses to the SSRI, escitalopram. Pharmacol, Biochem, Behav. 2004;77:391–8.
Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ss-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30:13513–24.
Turner EH, Loftis JM, Blackwell AD. Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharm Ther. 2006;109:325–38.
Thombre AG. Assessment of the feasibility of oral controlled release in an exploratory development setting. Drug Disco Today. 2005;10:1159–66.
Perry KW, Fuller RW. Extracellular 5-hydroxytryptamine concentration in rat hypothalamus after administration of fluoxetine plus L-5-hydroxytryptophan. J Pharm Pharm. 1993;45:759–61.
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8.
Holladay JW, Dewey MJ, Yoo SD. Pharmacokinetics and antidepressant activity of fluoxetine in transgenic mice with elevated serum alpha-1-acid glycoprotein levels. Drug Metab Dispos: Biol fate Chem. 1998;26:20–4.
Owens MJ, Krulewicz S, Simon JS, Sheehan DV, Thase ME, Carpenter DJ, et al. Estimates of serotonin and norepinephrine transporter inhibition in depressed patients treated with paroxetine or venlafaxine. Neuropsychopharmacology. 2008;33:3201–12.
Miro X, Perez-Torres S, Artigas F, Puigdomenech P, Palacios JM, Mengod G. Regulation of cAMP phosphodiesterase mRNAs expression in rat brain by acute and chronic fluoxetine treatment. An in situ hybridization study. Neuropharmacology. 2002;43:1148–57.
Oh JE, Zupan B, Gross S, Toth M. Paradoxical anxiogenic response of juvenile mice to fluoxetine. Neuropsychopharmacology. 2009;34:2197–207.
Haahr ME, Fisher PM, Jensen CG, Frokjaer VG, Mahon BM, Madsen K, et al. Central 5-HT4 receptor binding as biomarker of serotonergic tonus in humans: a [11C]SB207145 PET study. Mol Psychiatry. 2014;19:427–32.
Wegener G, Bandpey Z, Heiberg IL, Mork A, Rosenberg R. Increased extracellular serotonin level in rat hippocampus induced by chronic citalopram is augmented by subchronic lithium: neurochemical and behavioural studies in the rat. Psychopharmacology. 2003;166:188–94.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9.
Hodes GE, Hill-Smith TE, Suckow RF, Cooper TB, Lucki I. Sex-specific effects of chronic fluoxetine treatment on neuroplasticity and pharmacokinetics in mice. J Pharm Exp Ther. 2010;332:266–73.
Werling LL, Keller A, Frank JG, Nuwayhid SJ. A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder. Exp Neurol. 2007;207:248–57.
Jacobsen JP, Medvedev IO, Caron MG. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. 2012a;367:2444–59.
Berman RM, Marcus RN, Swanink R, McQuade RD, Carson WH, Corey-Lisle PK, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68:843–53.
Chen JW, Borgelt LM, Blackmer AB. Epidiolex (Cannabidiol): a new hope for patients with dravet or lennox-gastaut syndromes. Ann Pharmacother. 2019. 1060028018822124.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jacobsen, J.P.R., Oh, A., Bangle, R. et al. Slow-release delivery enhances the pharmacological properties of oral 5-hydroxytryptophan: mouse proof-of-concept. Neuropsychopharmacol. 44, 2082–2090 (2019). https://doi.org/10.1038/s41386-019-0400-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-019-0400-1