Abstract
The microbiota plays an essential role in the education, development, and function of the immune system, both locally and systemically. Emerging experimental and epidemiological evidence highlights a crucial cross-talk between the intestinal microbiota and the lungs, termed the ‘gut–lung axis’. Changes in the constituents of the gut microbiome, through either diet, disease or medical interventions (such as antibiotics) is linked with altered immune responses and homeostasis in the airways. The importance of the gut–lung axis has become more evident following the identification of several gut microbe-derived components and metabolites, such as short-chain fatty acids (SCFAs), as key mediators for setting the tone of the immune system. Recent studies have supported a role for SCFAs in influencing hematopoietic precursors in the bone marrow—a major site of innate and adaptive immune cell development. Here, we review the current understanding of host–microbe cross-talk along the gut–lung axis. We highlight the importance of SCFAs in shaping and promoting bone marrow hematopoiesis to resolve airway inflammation and to support a healthy homeostasis.
Similar content being viewed by others
Introduction
Our current understanding of the dynamics of host–microbe cross-talk and its implications for the immune system has been led by work on germ-free and antibiotics-treated mice.1,2 Microbial communities live in a mutualistic relationship with the host. Microbes benefit from a stable nutrient-rich microenvironment and in exchange, they perform important functions for the host including fermentation of dietary components for the generation of nutrients, vitamins, and metabolites.3 This relationship is fundamental for the development and education of the immune system, as well as for the maintenance of tissue and immune homeostasis. In fact, emerging evidence supports the importance of constitutive sensing of microbes and their products to tune the immune system towards a healthy homeostasis.4 Moreover, microbes provide local and systemic tonic signals to the innate and adaptive arms of the immune system supporting the development of protective responses against diverse pathogens.5,6
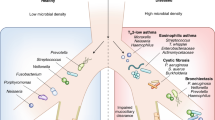
Disturbances in gut microbiota composition, as a result of genetic or exogenous factors including certain diets and antibiotic usage, is associated with a reduced capacity to mount adequate local and systemic immune responses.7 This gut dysbiosis in humans has been linked to inflammatory conditions in the gastrointestinal tract itself, but also in the airways, such as in asthma and chronic obstructive pulmonary disease (COPD).8,9 Of note, human epidemiological studies indicate microbial dysbiosis can have long-term consequences, which is supported by data from mouse models, where mice have an increased predisposition to allergic inflammation following early life antibiotic usage.10,11,12,13,14 Accumulating evidence has highlighted the influence of the gut microbiota on lung immunity, referred to as the gut–lung axis, though the underlying pathways and mechanisms are still areas of intensive research.15 Metabolic by-products derived from bacterial fermentation of dietary fibers have been reported as key local and systemic signaling molecules in sustaining immune and tissue homeostasis.16 The impact of various diets on lung health and disease has recently been reviewed.17
Short-chain fatty acids (SCFAs) are the most extensively studied metabolites, and have immunomodulatory functions upon diverse aspects of host physiology.18 Here, we summarize the current state of the field concerning the underlying mechanisms of SCFAs and the gut–lung axis. In particular, we will highlight the impact of SCFAs on the bone marrow, a primary lymphoid tissue involved in the generation and development of innate and adaptive immune cells.
The gut microbiota
The human body is colonized by a vast number of microbes including bacteria, fungi, archea, and protozoa, with the gut being the most densely colonized organ.19,20,21,22 Murine studies taking advantage of germ-free and antibiotic-treated mice have contributed tremendously to the concept of a host–microbe symbiosis in the gut, and its importance in local and systemic tissue homeostasis.1,2 For example, simply reconstituting the microbiome of such mice by fecal transplants is sufficient to restore mucosal immunity.23,24 Besides sustaining tissue homeostasis, ligands from commensal bacteria and their metabolic by-products can influence and fine-tune normal development and function of the mucosal immune system,25,26 and protect against bacterial and viral infections.27,28,29
Epidemiological studies have strongly correlated alterations in microbial communities with susceptibility to allergic airway diseases. For instance, reduced intestinal microbial diversity during infancy increased the risk of asthma development.30,31 Such constraints within gut bacterial communities are also observed following the use of broad-spectrum antibiotics, which are associated with predisposition to allergic airway diseases.32,33 In murine studies, antibiotic-driven depletion of certain bacterial species of the gut microbiome increases not only predisposition to airway diseases but also pulmonary viral infections.28,34
Thus, variations in microbial composition of the gut microbiota perturb cross-talk with the host and can have profound influences upon immune responses and disease susceptibility.35 At present, a great deal of effort is being put into the identification of bacterial communities associated with health and disease in humans.35,36 Although metagenomic sequencing of samples, an approach where DNA is sequenced directly from samples without prior amplification, is rapidly advancing, investigations of microbial communities have commonly been based on sequencing variable regions of the 16S ribosomal RNA (rRNA) gene. Taxonomic identification, ranging from domain to genus and species of bacteria, is based on sequence similarities of 16S rRNA gene amplicons against a reference database. These approaches have unveiled the exceptional complexity and diversity of microbial communities and revealed a spatial partitioning of commensal communities determined by the microenvironment at different body sites. Thus, distinct anatomical sites (habitats) host unique microbial communities.37
The human body is estimated to be colonized by around 38 trillion bacteria (3.8 × 1013).38 The gastrointestinal tract, which is the most extensively and diversely colonized organ, is believed to harbor between 100 thousand and 100 billion bacteria per mL of luminal content (105–1011) depending on the region.22,38 The abundance and diversity of microbial communities increases along the gut, with the colon harboring the densest and most metabolically active populations.39 The diversity of the human gut microbiome shows interpersonal variations, which are due to genetics but also diverse environmental factors, such as lifestyle and diet.40,41,42 Despite these variations across individuals and site-specificity of the intestinal microbiota, 16S rRNA and metagenomics sequencing allowed the description of a ‘core’ microbiome in the healthy gastrointestinal tract that was dominated by the bacterial phyla Firmicutes and Bacteroidetes.40,43,44
The airway microbiota
In comparison to the intestinal microbiota, studies on the lung microbiome are still in their infancy. The lower respiratory tract was historically considered to be ‘sterile’, mostly due to the failure to grow lung microbes in routine microbiological cultures from healthy individuals. This dogma was contested with advances in sequencing techniques that were able to detect microbial DNA in the lungs of individuals, even under healthy steady-state conditions.45 Nevertheless, technical limitations such as sampling method, oropharyngeal cross-contamination during collection, and low microbial loads have challenged the identification and discrimination of a resident microbiome as compared to a transiently present bacterial community in the lower airways. This is an area of intensive investigation that is slowly shedding light on the importance of direct host–microbe interactions in the airways.46
The composition of the microbiota differs significantly between the upper and lower respiratory tract in healthy individuals, questioning if samples of the upper airways can reflect the microbiome in the lower respiratory tract.47 The prevalence of distinct bacterial species in these compartments supports the concept of niche-specific microbial colonization at distinct anatomical sites.48 Nonetheless, some bacterial communities are shared between the lung and the oral cavity although at different abundances, suggesting that the lung microbial community is partially seeded through microaspiration of the oral microbiome.45,49,50 A comparative study on microbial communities in the lower airways and the oral cavity of non-smoking and smoking individuals has contributed to the identification of a ‘healthy’ lower respiratory tract microbiome. The overall bacterial communities in the lung resemble those in the oral cavity with Streptococcus, Prevotella, and Veillonella being the most common genera.45 The predominant phyla in the lungs of healthy individuals are Bacteroidetes and Firmicutes, while the oropharynx is dominated by Firmicutes, Proteobacteria, and Bacteriodetes.45,48,50,51 By contrast, the nasal microbial community in healthy individuals is reported to be closer to the skin microbiota as Firmicutes and Actinobacteria phyla are the most prevalent.48,52,53
Studies by our group have indicated that the airway microbiome forms rapidly post-birth, reaching its mature diversity within 2–3 post-natal weeks in mice54 and 2–3 post-natal months in humans.55 In mice, formation of the airway microbiome coincides with immune maturation steps that underlie the development of tolerance to inhaled allergens.54 In humans, it is not feasible to track the lower airway microbiome longitudinally, however, the constituents of the microbiome associated with distinct steps of immune maturation indicative of ongoing cross-talk between the airway resident microbes and immune cells.56,57
Perturbation of the gut–lung axis during dysbiosis
Immune homeostasis is dependent on a microbiome that provides cues, including microbial components and metabolites, for appropriate maturation and priming of the immune system.5 In humans, environmental factors, such as diet, antibiotic treatment, and stress can shift the gut microbiota towards decreased abundance of beneficial bacterial species accompanied by outgrowth of pathogenic ones.58 This perturbation in microbial composition and function, referred to as dysbiosis, disrupts tissue and immune homeostasis and is associated with diverse inflammatory diseases within and outside the gastrointestinal tract.59 For example, disruption of intestinal–pulmonary cross-talk is linked to increased susceptibility to airway diseases and infections, including allergies.60 The importance of the gut–lung axis is exemplified in patients with chronic gastrointestinal diseases, such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD), who have a higher prevalence of pulmonary diseases.60,61,62
Murine and human studies linked antibiotic use early in life to gut microbiota disruption and increased asthma risk.11,13,34 In human neonates, reduced abundance of bacteria, such as Bifidobacteria, Akkermansia, and Faecalibacteria in the intestinal tract has been linked with an enhanced risk of developing atopy and asthma.63,64 These data suggest the existence of a critical developmental window early in life where microbial diversity in the gastrointestinal tract primes systemic immune responses towards health in mammals.11,12,30 Besides allergic airway diseases, murine studies showed that the gut microbiota also plays a protective role against bacterial and viral pulmonary infections by regulating innate and adaptive immune responses.28,65,66
Although most evidence indicates the primary direction of cross-talk occurs from the gut to the lung, there remains the possibility of communication in the opposite direction. Chronic lung disorders, such as asthma, COPD, and cystic fibrosis (CF) exhibit not only a dysbiotic airway microbiota but also components of gastrointestinal perturbation such as IBS.9,67,68 Moreover, respiratory influenza infections in mice can indirectly induce intestinal immune injury and alter the intestinal microbiota. The resulting gut dysbiosis promotes inflammation through the outgrowth of Enterobacteriaceae and the reduction of Lactobacilli and Lactococci.69
Overall, there is a clear cross-talk between the gut and the lungs, which is vital for maintaining homeostasis and educating the host immune system. The mechanisms through which the gut impacts on lung health or disease and vice versa are only starting to be uncovered.70 Several factors have been shown to exert their functions along the gut–lung axis including systemic dissemination of bacterial-derived components and metabolic degradation products, with SCFAs being the most prominent immunomodulatory metabolites.
Short chain fatty acids—prototypic immunomodulatory bacterial metabolites
The identification of microbial communities that dictate health and disease across individuals and populations, is an active area of research.71,72 Diverse exogenous and endogenous factors such as genetics, age, and diet are known to impact on the composition of the gut microbiota.71,73 Diet plays one of the most determinant roles in shaping the gut microbiome and represents one of the easiest and most attractive options for therapeutic interventions.74 In a comparative study between African and European diets in children, the African diet, which is high in fiber and low in animal protein and fat, was linked with an enrichment of the genus Prevotella in the gut, while Bacteroides dominated in the high-protein and high-fat ‘Western’ diet. The African high-fiber diet supported the expansion of bacteria such as Prevotella and Xylanibacter that carry genes capable of fermenting fiber leading to the production of SCFA (e.g. acetate, butyrate, and propionate), known for their anti-inflammatory activity.75,76,77
The importance of SCFAs levels in the local and peripheral milieu is exemplified by their pleiotropic functions in mice ranging from maintaining and reinforcing intestinal–epithelial integrity to dampening inflammation in the gut and respiratory tract.16,78,79 How commensal-derived SCFAs link the gut with the lung is only starting to be uncovered80; however, it is clearly multifaceted with SCFAs being able to influence directly or indirectly the function of various cells including epithelial cells, innate and adaptive immune cells.
Thus far, two main signaling pathways have been ascribed to the underlying mechanisms of SCFAs.
The most prominent direct effect of SCFAs on host immunity is through engagement of G protein-coupled receptors (GPCRs), which are differentially expressed by several cell types and tissues.81 These GPCRs include GPR43 (also known as free fatty acid receptor 2, FFAR2), GPR41 (FFAR3), and GPR109A (NIACR1).81 Moreover, GPCRs are coupled to distinct downstream effector molecules, thus adding another level of complexity to SCFA-induced GPCR signal transduction that can result in different outcomes on cellular functions and responses in different cell types. Signaling through these transmembrane receptors linked to regular G proteins is associated with an inflammatory effect through activation of mitogen-activated protein (MAP) kinases, phosphoinositide 3 (PI3K)-kinases, and mammalian target of rapamycin (mTOR).82 Alternatively, signal transduction pathways involving the intracellular ß-arrestins drives an anti-inflammatory response.82,83,84 GPR41 and GPR43 are coupled to Gi/o or Gq and can promote a pro-inflammatory program through MAPK activation, while GPR43 can engage an alternative anti-inflammatory signaling pathway through the activation of ß-arrestin2 that inhibits NF-κB activity.84,85 However, it is not entirely clear which GPCR signaling pathways are preferentially engaged under distinct conditions by SCFAs and if they interact with each other to fine-tune immune responses. An additional documented downstream signaling effect of SCFAs is the modulation of immune responses through inhibition of histone deacetylase (HDAC) activity in various cell86 types. SCFAs can exert their suppressive effect upon cell entry via passive diffusion, through absorption via the high-affinity Na+-coupled monocarboxylate transporter SLC5A8 or the low-affinity H+-coupled carrier SLC16A1.87,88,89
Thus, SCFAs have pleiotropic functions in diverse cell types and tissues. Their activity depends not only on their relative availability and affinity to the receptors, but also on GPCR expression, availability of transporter molecules, and downstream effector molecules in various cells types.5,81
Immune modulation of the intestinal tract and airways by SCFAs
To maintain a homeostatic host–microbe relationship in the gastrointestinal tract, direct contact between epithelial cells and colonizing microbes are minimized through various barrier defense mechanisms including mucus production, secretion of immunoglobulin A (sIgA), and of antimicrobial peptides.90,91 SCFAs can modulate diverse aspects of these defense lines to maintain mucosal immunity. For example, SCFAs were shown to enhance intestinal epithelial barrier function (IEC) through increasing differentiation and mucus production by goblet cells92,93 and fortifying tight junction permeability.94 Moreover, SCFAs promote intestinal IgA production through enhancing plasma B cell metabolism, and differentiation to protect the gut from inflammation.95,96 SCFAs can also signal through GPR43 and GPR109A on intestinal epithelial cells to induce NLRP3 inflammasome activation that is an essential cell survival and repair mechanism, and that prevents against dextran sulfate sodium (DSS)-induced colitis.97
SCFAs also promote anti-inflammatory mechanisms to sustain intestinal homeostasis.98 For instance, butyrate stimulates anti-inflammatory signaling to suppress intestinal inflammation and colon cancer. It can signal through GPR109A on colonic macrophages and dendritic cells (DCs) to induce IL-10 production, which in turn elicit the differentiation of immune-suppressive IL-10-producing T cells and regulatory T (Treg) cells.99,100 In addition, butyrate has been reported to support IL-18 production in colonic epithelium in a GPR109A-dependent manner,100 which is known to suppress colonic inflammation and inflammation-associated cancers101. In addition, SCFAs can regulate intestinal inflammation through induction of colonic forkhead box P3 (Foxp3)+ Treg cell differentiation and function in a GPR43-dependent manner. Through their HDAC inhibitor activity, SCFAs enhance histone H3 acetylation at the Foxp3 locus that is associated with a permissive chromatin structure, thus increasing gene accessibility for transcription.102,103
A recent report demonstrated that SCFAs can promote gut homeostasis through a positive feedback loop by directing the metabolism of colonic epithelial cells towards fatty acid ß-oxydation (FAO).104
Under homeostatic conditions, microbiota-derived butyrate is sensed by the nuclear protein peroxisome proliferator-activated receptor gamma (PPARγ) in colonic epithelial cells.105 Signaling through this transcription factor directs the energy metabolism of colonocytes to FAO and oxidative phosphorylation (OXPHOS) in mitochondria.106 These two metabolic pathways require high oxygen consumption resulting in epithelial hypoxia.104,107 This anaerobic state (anaerobiosis) in the lumen of the colon ensures the growth of a balanced SCFA-producing microbiota community dominated by obligate anaerobic bacteria of the phyla Firmicutes and Bacteroides. Concomitantly, epithelial hypoxia prevents the outgrowth of dysbiotic bacterial communities, such as facultative anaerobic bacteria of the phylum Proteobacteria (Enterobacteriaceae), a hallmark of gut dysbiosis.104,107,108
The protective role of SCFAs has been extensively studied in the murine gut. Accumulating evidence supports an anti-inflammatory and immune-modulatory action of SCFAs also in the periphery, in particular along the gut–lung axis.16,79 Similar to the intestinal tract, SCFAs are able to generate an extrathymic peripheral Treg cell pool, linked to dampening allergic airway diseases through HDAC inhibition.109 SCFAs can promote T cell differentiation into T helper (Th) 1 and Th17 effector cells and IL-10+Tregs, independent of GPR41 and GPR43. In this case, SCFAs act as HDAC inhibitors to enhance the mTOR–S6K pathway required for T cell differentiation and cytokine expression.110 Recently, SCFAs were also shown to serve as a substrate for FAO to significantly enhance cellular metabolism and function of CD8+T cells during influenza infection. These metabolic and functional changes in CD8+T cells were predominantly GPR41-dependent.111
Effects of SCFAs on bone marrow hematopoiesis and airway immunity
The molecular mechanism underlying the protective effect of gut microbes and commensal-derived metabolites on lung immunity has been a research area of great interest in recent years. Emerging evidence, particularly involving SCFAs, has highlighted a link between the bone marrow, the gut, and the airways.
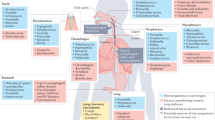
There are limited reports of SCFAs being detected in the lungs, suggesting that circulating SCFAs do not accumulate in the tissue and that lung-resident bacteria do not produce SCFAs in substantial amounts, possibly due to the absence of substrates. The role of SCFAs directly in the airways is possible, but likely to be negligible, and rather it is their effects on immune cells in the periphery with subsequent recruitment to the lungs that underlies their promotion of lung homeostasis and immunity. Recent work from our laboratory has provided a mechanistic explanation through which gut-derived SCFAs elicit their protective mechanisms against allergic airway diseases and respiratory infection.79 Circulatory acetate or propionatemodulated DC hematopoiesis and functionality in the bone marrow during Th2 cell-mediated allergic airway inflammation (AAI). The metabolites were able to enhance generation of macrophage and DC progenitors (MDPs), and the more differentiated common DC progenitors (CDPs) in the bone marrow.79,112 These DC precursor cells subsequently populated the lungs where they matured into CD11b+ DCs that were inefficient at allergen presentation and consequently in activating Th2 effector cells.113,114 Thus, AAI could not be sustained and was resolved quickly79 (Fig. 1). In more recent work, we have shown that the effect of SCFAs on bone marrow hematopoiesis and progenitor cell commitment was context-dependent. During influenza infection, SCFAs only influenced the MDP subsets, while other hematopoietic progenitors were unaffected.111 MDPs commit into either CDPs or monocytes, the latter consisting of two subsets distinguished by Ly6C (also referred to as Gr-1) expression.115 Upon inflammatory conditions, Ly6C+ monocytes can give rise to inflammatory macrophages or DCs that can cause immunopathology.115,116 By contrast, Ly6C− monocytes can differentiate into alternatively activated macrophages (AAMs) in the lungs, endowed with anti-inflammatory and tissue repair capacities.117,118 Butyrate or propionate enhanced MDP precursors and their commitment into Ly6C− monocytes without affecting the Ly6C+ subset in a GPR41-dependent manner during influenza infection. In the lungs, these Ly6C− monocytes adopted an AAM cell fate that had limited capacity to express the neutrophil chemoattractant CXCL1.111,119 Thus, AAMs limited the influx of neutrophils into the airways and resolved neutrophil-associated immunopathologies during viral infections120,111 (Fig. 1). These data suggest that along the gut–lung axis, although context-dependent, SCFAs prime myeloid cells in the bone marrow, which subsequently migrate to the lungs and shape an anti-inflammatory milieu.79,111
Effect of SCFAs on bone marrow hematopoiesis. Fermentation of undigested dietary fibers by the gut microbiota results in the production of short-chain fatty acids (SCFAs), with propionate, acetate, and butyrate as the most abundant metabolites. SCFAs disseminate from the gut into the blood stream where they can reach the bone marrow (BM) to promote hematopoiesis. In the bone marrow, hematopoietic stem cells (HSCs) can differentiate into multipotent progenitors (MPPs) which in turn can commit to common lymphoid precursors (CLPs) or to common myeloid precursors (CMPs). CMPs can further differentiate into granulocyte and macrophage progenitors (GMPs) that commit to monocyte and DC progenitors (MDPs). MDPs can give rise to Ly6C− and Ly6C+ monocytes or to common DC precursors (CDPs). Monocytes can leave the BM and circulate as patrolling Ly6C− or inflammatory Ly6C+ monocytes in the periphery. Under inflammatory conditions, Ly6C+ monocytes can give rise to CD11b high monocyte-derived DCs (moDCs) in the lungs. CDPs differentiate into pre-classical DCs (pre-DCs) which then migrate from the bone marrow into the lungs where they can mature into CD103+ DCs or CD11b+ conventional DCs (cDCs). Ly6C− monocytes can differentiate into alternatively activated macrophages (AAMs). During house dust mite (HDM)-induced allergic airway inflammation, propionate and acetate were shown to enhance generation of MDPs and CDPs. These inflammatory DC precursor cells migrate to the lung where they can mature into CD11b high DCs that have a high phagocytic capacity but are less activated as seen by reduced expression of CD40, PD-L2, and CD86. Thus, these lung DCs have a reduced ability to induce T helper 2 (Th2) effector function and proliferation, and can therefore not sustain a Th2 cell-mediated allergic airway inflammation. During influenza infection, butyrate or propionate increase the proliferation of MDPs resulting in increased Ly6C− monocytes in the bone marrow and in the lungs. These patrolling Ly6C− monocytes can differentiate into AAMs that express lower levels of CXCL1, a neutrophil chemoattractant. Thus, neutrophil influx is reduced resulting in decreased influenza-mediated lung immunopathology. Moreover, SCFAs directly promote the activation of influenza-specific CD8+ T cells by enhancing their metabolism, and consequently their antiviral activity
Induction of bone marrow myelopoiesis by microbial components
Commitment of hematopoietic stem cells (HSCs) to lymphoid or myeloid lineages via a series of progenitor cells, takes place in the bone marrow and requires intrinsic and extrinsic cues, such as growth factors and cytokines.121,122 In addition to microbial metabolites, it has long been known that the intestinal microbiota itself also influences hematopoiesis in the bone marrow.123,124 The microbiota has been reported to be specifically involved in the promotion of steady-state myelopoiesis, while differentiation potential of HSCs was unaltered.124,125,126 Moreover, the complexity of the intestinal microbiota dictates the size of the myeloid pool in the bone marrow.125,127 So far, these studies uncovered a specific role for the intestinal microbiome on maintaining and enhancing differentiation potential of granulocyte and macrophage progenitor (GMP) populations as recolonization of germ-free mice restored defects in GMP-derived myeloid cells. Consequently, differentiation of GMP-derived myeloid cells can give rise to certain peripheral tissue-resident granulocytes that are essential sentinels for promoting protection against systemic bacterial infections such as L. monocytogenes.124,125,127 In contrast, Josefsdottir and colleagues reported that broad-spectrum antibiotic therapy depleted mainly HSCs and multipotent progenitors (MPPs), while myeloid cells were maintained at normal levels in the bone marrow.128 This discrepancy could be due to differences in duration and nature of the antibiotics regimen and requires further investigation for clarification.
The microbiota contains diverse microbial components and metabolites that can influence bone marrow hematopoiesis. Multipotent HSCs and progenitors express Toll-like receptors (TLRs) on their membranes, suggesting that direct sensing of extracellular microbial-associated molecular patterns (MAMPs) are involved in the maintenance of steady-state hematopoiesis.129 Indeed, Nagai and colleagues demonstrated that signaling through TLR2 and TLR4 controls proliferation and differentiation of HSCs and of the myeloid progenitors common myeloid precursors (CMPs) and GMPs, independently of growth and differentiation factors under homeostatic conditions.129,130 Moreover, the authors described the essential role of the TLR adapter molecule myeloid differentiation primary response 88 (MyD88) in terminal differentiation of granulocytes in the bone marrow.129,130 This finding was supported by studies identifying the essential role of TLRs via MyD88 signaling in driving steady-state myelopoiesis that generates a reserve pool of myeloid cells in the bone marrow.125,127 Hence, MyD88-deficient mice phenocopied germ-free mice and presented reduced myeloid and GMP populations.131,132
Similar to SCFAs, TLR agonists seem to influence bone marrow hematopoiesis at the myeloid stage that is required for protection against infection. More work is needed to understand the mechanisms, and context, for how SCFAs selectively prime myeloid precursors in the bone upon inflammation and if this also holds true during homeostasis. Additional studies on the underlying mechanisms could give valuable insight on how to modulate the immune system at one of its most fundamental levels for therapeutic purposes.
Concluding remarks
Advances in understanding host–microbe mutualism have highlighted the importance of gut microbe components and metabolites in maintaining tissue and immune homeostasis.25,133,134 Among these commensal-derived metabolites, SCFAs have emerged as key signaling molecules within the gut and in the periphery to limit inflammation and direct protective responses.16 The level of local and systemic SCFA is highly dependent on the fermentable fiber content in diets and on the local microbial community capable of fermenting these fibers. Likewise, dietary fibers can shape the gut microbiome by altering the Firmicutes to Bacteroidetes ratio, allowing outgrowth of Bacteroidetes that have increased SCFA fermentation capacity.79,135 Given their protective effects against intestinal and respiratory inflammatory diseases, SCFAs and diets represent a promising therapeutic approach. Better understanding of the molecular and cellular mechanisms underlying SCFA actions, which seem to be context-dependent, will ultimately improve the potential of therapeutic interventions.
The concept of the gut–bone marrow–lung axis has gained increasing attention with the discovery of the influence of SCFAs on bone marrow hematopoiesis. As found for the gut microbiota, SCFAs seem to influence myelopoiesis, in particular. During allergic airway diseases, SCFAs induce the commitment of MDPs into immature DCs that are unable to sustain Th2 responses, while they promote the differentiation of MDPs into patrolling Ly6C− monocytes that limit neutrophil-induced immunopathology upon influenza infection. Thus far, SCFAs induce myelopoiesis in both contexts to generate an anti-inflammatory milieu in the airways.79,111 Further studies will clarify if SCFAs maintain their preference for myelopoiesis also in other chronic inflammatory settings such as cancer and autoimmunity.
Hematopoiesis also occurs outside the bone marrow, referred to as extramedullary hematopoiesis. This process takes place in the spleen or liver and is induced upon immune responses against pathogens to initiate DC and phagocyte production.136,137 The gut microbiota was reported to promote hematopoiesis of myeloid progenitors in the spleen.127 However, the potential role of SCFAs in extramedullary hematopoiesis remains to be assessed.
Taken together, the gut–bone marrow–lung axis represents a mechanistic explanation for how the gut microbiota and gut-derived SCFAs regulate hematopoiesis to prime the immune system against infections and allergic responses in the periphery.79,111 This pathway holds great potential for future therapies, but given the pleiotropic effects of microbial metabolites, and the fundamental implications of influencing the immune system at the level of hematopoiesis, further studies are warranted.
References
Gensollen, T. et al. How colonization by microbiota in early life shapes the immune system. Science 352, 539–544 (2016).
Wostmann, B. S. The germfree animal in nutritional studies. Annu. Rev. Nutr. 1, 257–279 (1981).
Krajmalnik-Brown, R. et al. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 27, 201–214 (2012).
Budden, K. F. et al. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 15, 55–63 (2017).
Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Belkaid, Y. & Harrison, O. J. Homeostatic immunity and the microbiota. Immunity 46, 562–576 (2017).
Willing, B. P., Russell, S. L. & Finlay, B. B. Shifting the balance: antibiotic effects on host–microbiota mutualism. Nat. Rev. Microbiol. 9, 233–243 (2011).
Rapozo, D. C., Bernardazzi, C. & de Souza, H. S. Diet and microbiota in inflammatory bowel disease: the gut in disharmony. World J. Gastroenterol. 23, 2124–2140 (2017).
Rutten, E. P. A. et al. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest 145, 245–252 (2014).
Noverr, M. C. et al. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect. Immun. 73, 30–38 (2005).
Russell, S. L. et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 13, 440–447 (2012).
Russell, S. L. et al. Perinatal antibiotic-induced shifts in gut microbiota have differential effects on inflammatory lung diseases. J. Allergy Clin. Immunol. 135, 100–109 (2015).
Korpela, K. et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat. Commun. 7, 10410 (2016).
Risnes, K. R. et al. Antibiotic exposure by 6 months and asthma and allergy at 6 years: findings in a cohort of 1,401 US children. Am. J. Epidemiol. 173, 310–318 (2011).
Marsland, B. J., Trompette, A. & Gollwitzer, E. S. The gut–lung axis in respiratory disease. Ann. Am. Thorac. Soc. 12(Suppl. 2), S150–S156 (2015).
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Wypych, T. P., Marsland, B. J. & Ubags, N. D. J. The impact of diet on immunity and respiratory diseases. Ann. Am. Thorac. Soc. 14(Suppl. 5), S339–S347 (2017).
Koh, A. et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Grice, E. A. & Segre, J. A. The human microbiome: our second genome. Annu. Rev. Genom. Hum. Genet. 13, 151–170 (2012).
Ley, R. E., Peterson, D. A. & Gordon, J. I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124, 837–848 (2006).
Eckburg, P. B., Lepp, P. W. & Relman, D. A. Archaea and their potential role in human disease. Infect. Immun. 71, 591–596 (2003).
Sender, R., Fuchs, S. & Milo, R. Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell 164, 337–340 (2016).
Umesaki, Y. et al. Segmented filamentous bacteria are indigenous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol. Immunol. 39, 555–562 (1995).
Ekmekciu, I. et al. Immune responses to broad-spectrum antibiotic treatment and fecal microbiota transplantation in mice. Front. Immunol. 8, 397 (2017).
Mazmanian, S. K. et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118 (2005).
Rakoff-Nahoum, S. et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Abt, M. C. et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37, 158–170 (2012).
Ichinohe, T. et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl Acad. Sci. USA 108, 5354–5359 (2011).
Franchi, L. et al. NLRC4-driven production of IL-1beta discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat. Immunol. 13, 449–456 (2012).
Abrahamsson, T. R. et al. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 44, 842–850 (2014).
Bisgaard, H. et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 128, 646–652 e1–5 (2011).
Marra, F. et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics 123, 1003–1010 (2009).
Metsala, J. et al. Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clin. Exp. Allergy 45, 137–145 (2015).
Russell, S. L. et al. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes 4, 158–164 (2013).
Lozupone, C. A. et al. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Greenhalgh, K. et al. The human gut microbiome in health: establishment and resilience of microbiota over a lifetime. Environ. Microbiol. 18, 2103–2116 (2016).
Costello, E. K. et al. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697 (2009).
Sender, R., Fuchs, S. & Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14, e1002533 (2016).
Donaldson, G. P., Lee, S. M. & Mazmanian, S. K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14, 20–32 (2016).
Eckburg, P. B. et al. Diversity of the human intestinal microbial flora. Science 308, 1635–1638 (2005).
Lay, C. et al. Colonic microbiota signatures across five northern European countries. Appl. Environ. Microbiol. 71, 4153–4155 (2005).
Voreades, N., Kozil, A. & Weir, T. L. Diet and the development of the human intestinal microbiome. Front. Microbiol. 5, 494 (2014).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Zhernakova, A. et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352, 565–569 (2016).
Morris, A. et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am. J. Respir. Crit. Care Med. 187, 1067–1075 (2013).
Marsland, B. J. & Gollwitzer, E. S. Host–microorganism interactions in lung diseases. Nat. Rev. Immunol. 14, 827–835 (2014).
Goddard, A. F. et al. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc. Natl Acad. Sci. USA 109, 13769–13774 (2012).
Lemon, K. P. et al. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. mBio 1, e00129–10 (2010).
Bassis, C. M. et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 6, e00037 (2015).
Dickson, R. P. et al. The microbiome and the respiratory tract. Annu. Rev. Physiol. 78, 481–504 (2016).
Dewhirst, F. E. et al. The human oral microbiome. J. Bacteriol. 192, 5002–5017 (2010).
Rasmussen, T. T. et al. Resident aerobic microbiota of the adult human nasal cavity. APMIS 108, 663–675 (2000).
Grice, E. A. & Segre, J. A. The skin microbiome. Nat. Rev. Microbiol. 9, 244–253 (2011).
Gollwitzer, E. S. et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med. 20, 642–647 (2014).
Pattaroni, C. et al. Early-life formation of the microbial and immunological environment of the human airways. Cell Host Microbe 24, 857–865.e4 (2018).
Mathieu, E. et al. Paradigms of lung microbiota functions in health and disease, particularly, in asthma. Front. Physiol. 9, 1168 (2018).
Huffnagle, G. B., Dickson, R. P. & Lukacs, N. W. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol. 10, 299–306 (2017).
Hakansson, A. & Molin, G. Gut microbiota and inflammation. Nutrients 3, 637–682 (2011).
Shreiner, A. B., Kao, J. Y. & Young, V. B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 31, 69–75 (2015).
Keely, S., Talley, N. J. & Hansbro, P. M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 5, 7–18 (2012).
Wang, H. et al. Gut–lung crosstalk in pulmonary involvement with inflammatory bowel diseases. World J. Gastroenterol. 19, 6794–6804 (2013).
Yazar, A. et al. Respiratory symptoms and pulmonary functional changes in patients with irritable bowel syndrome. Am. J. Gastroenterol. 96, 1511–1516 (2001).
Fujimura, K. E. et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 22, 1187–1191 (2016).
Kalliomaki, M. et al. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 107, 129–134 (2001).
Chen, L. W., Chen, P. H. & Hsu, C. M. Commensal microflora contribute to host defense against Escherichia coli pneumonia through Toll-like receptors. Shock 36, 67–75 (2011).
Schuijt, T. J. et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65, 575–583 (2016).
Baral, V. & Connett, G. Acute intestinal obstruction as a presentation of cystic fibrosis in infancy. J. Cyst. Fibros. 7, 277–279 (2008).
Roussos, A. et al. Increased prevalence of irritable bowel syndrome in patients with bronchial asthma. Respir. Med. 97, 75–79 (2003).
Wang, J. et al. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J. Exp. Med. 211, 2397–2410 (2014).
Tulic, M. K., Piche, T. & Verhasselt, V. Lung–gut cross-talk: evidence, mechanisms and implications for the mucosal inflammatory diseases. Clin. Exp. Allergy 46, 519–528 (2016).
Turnbaugh, P. J. et al. The human microbiome project. Nature 449, 804–810 (2007).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Xu, Z. & Knight, R. Dietary effects on human gut microbiome diversity. Br. J. Nutr. 113(Suppl.), S1–S5 (2015).
Donovan, S. M. Introduction to the special focus issue on the impact of diet on gut microbiota composition and function and future opportunities for nutritional modulation of the gut microbiome to improve human health. Gut Microbes 8, 75–81 (2017).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Chen, T. et al. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 7, 2594. (2017).
Fukuda, S. et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 (2011).
Trompette, A. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166 (2014).
Sharon, G. et al. Specialized metabolites from the microbiome in health and disease. Cell. Metab. 20, 719–730 (2014).
Husted, A. S. et al. GPCR-mediated signaling of metabolites. Cell. Metab. 25, 777–796 (2017).
Thorburn, A. N., Macia, L. & Mackay, C. R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40, 833–842 (2014).
Li, M. et al. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 831, 52–59 (2018).
Gao, H. et al. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol. Cell 14, 303–317 (2004).
Kim, M. H. et al. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145, 396–406 (2013). e1–10.
Thorburn, A. N. et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 6, 7320 (2015).
Miyauchi, S. et al. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na(+)-coupled transporter for short-chain fatty acids. J. Biol. Chem. 279, 13293–13296 (2004).
Gurav, A. et al. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem. J. 469, 267–278 (2015).
Sivaprakasam, S. et al. Short-chain fatty acid transporters: role in colonic homeostasis. Compr. Physiol. 8, 299–314 (2017).
Hooper, L. V. & Macpherson, A. J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 10, 159–169 (2010).
McGuckin, M. A. et al. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 9, 265–278 (2011).
Gaudier, E. et al. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1168–G1174 (2004).
Willemsen, L. E. et al. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 52, 1442–1447 (2003).
Ohata, A., Usami, M. & Miyoshi, M. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition 21, 838–847 (2005).
Kim, M. et al. Gut Microbial metabolites fuel host antibody responses. Cell Host Microbe 20, 202–214 (2016).
Wu, W. et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 10, 946–956 (2017).
Macia, L. et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 6, 6734 (2015).
Donohoe, D. R. et al. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 48, 612–626 (2012).
Geuking, M. B. et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34, 794–806 (2011).
Singh, N. et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139 (2014).
Dupaul-Chicoine, J. et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32, 367–378 (2010).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Byndloss, M. X. et al. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575 (2017).
Alex, S. et al. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor gamma. Mol. Cell. Biol. 33, 1303–1316 (2013).
Varga, T., Czimmerer, Z. & Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 1812, 1007–1022 (2011).
Litvak, Y., Byndloss, M. X. & Baumler, A. J. Colonocyte metabolism shapes the gut microbiota. Science 362, 6418 (2018).
Rigottier-Gois, L. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis. Isme. J. 7, 1256–1261 (2013).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Park, J. et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 8, 80–93 (2015).
Trompette, A. et al. Dietary fiber confers protection against flu by shaping Ly6c(−) patrolling monocyte hematopoiesis and CD8(+) T cell metabolism. Immunity 48, 992–1005.e8 (2018).
Liu, K. et al. In vivo analysis of dendritic cell development and homeostasis. Science 324, 392–397 (2009).
Kopf, M., Schneider, C. & Nobs, S. P. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 16, 36–44 (2015).
Desch, A. N., Henson, P. M. & Jakubzick, C. V. Pulmonary dendritic cell development and antigen acquisition. Immunol. Res. 55, 178–186 (2013).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Fogg, D. K. et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311, 83–87 (2006).
Landsman, L. & Jung, S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J. Immunol. 179, 3488–3494 (2007).
Landsman, L., Varol, C. & Jung, S. Distinct differentiation potential of blood monocyte subsets in the lung. J. Immunol. 178, 2000–2007 (2007).
Labonte, A. C., Tosello-Trampont, A. C. & Hahn, Y. S. The role of macrophage polarization in infectious and inflammatory diseases. Mol. Cells 37, 275–285 (2014).
Shirey, K. A. et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent. Mucosal Immunol. 3, 291–300 (2010).
Rosmarin, A. G., Yang, Z. & Resendes, K. K. Transcriptional regulation in myelopoiesis: hematopoietic fate choice, myeloid differentiation, and leukemogenesis. Exp. Hematol. 33, 131–143 (2005).
Metcalf, D. Hematopoietic cytokines. Blood 111, 485–491 (2008).
Iwamura, C. et al. Sensing of the microbiota by NOD1 in mesenchymal stromal cells regulates murine hematopoiesis. Blood 129, 171–176 (2017).
Tada, T. et al. Level of myelopoiesis in the bone marrow is influenced by intestinal flora. Cell. Immunol. 173, 155–161 (1996).
Balmer, M. L. et al. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J. Immunol. 193, 5273–5283 (2014).
Deshmukh, H. S. et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat. Med. 20, 524–530 (2014).
Khosravi, A. et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 15, 374–381 (2014).
Josefsdottir, K. S. et al. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood 129, 729–739 (2017).
Nagai, Y. et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 24, 801–812 (2006).
Takizawa, H. et al. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J. Exp. Med. 208, 273–284 (2011).
Fiedler, K. et al. MyD88 is involved in myeloid as well as lymphoid hematopoiesis independent of the presence of a pathogen. Am. J. Blood Res. 3, 124–140 (2013).
Yanez, A. et al. Detection of a TLR2 agonist by hematopoietic stem and progenitor cells impacts the function of the macrophages they produce. Eur. J. Immunol. 43, 2114–2125 (2013).
Belkaid, Y. & Naik, S. Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immunol. 14, 646–653 (2013).
Bernard, H. et al. Dietary pectin-derived acidic oligosaccharides improve the pulmonary bacterial clearance of Pseudomonas aeruginosa lung infection in mice by modulating intestinal microbiota and immunity. J. Infect. Dis. 211, 156–165 (2015).
Holscher, H. D. et al. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: follow-up of a randomized controlled trial. Am. J. Clin. Nutr. 101, 55–64 (2015).
Kim, C. H. Homeostatic and pathogenic extramedullary hematopoiesis. J. Blood Med. 1, 13–19 (2010).
Robbins, C. S. et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 125, 364–374 (2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dang, A.T., Marsland, B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol 12, 843–850 (2019). https://doi.org/10.1038/s41385-019-0160-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-019-0160-6
This article is cited by
-
Vitamin D and the microbiota connection: understanding its potential to improve COPD outcomes
The Egyptian Journal of Bronchology (2024)
-
Pathophysiology of acute lung injury in patients with acute brain injury: the triple-hit hypothesis
Critical Care (2024)
-
Esmolol increases the fecal abundance of Lactobacillus in a rat model of sepsis
Intensive Care Medicine Experimental (2024)
-
Exploring the immune-inflammatory mechanism of Maxing Shigan Decoction in treating influenza virus A-induced pneumonia based on an integrated strategy of single-cell transcriptomics and systems biology
European Journal of Medical Research (2024)
-
Application of Microbiome-Based Therapies in Chronic Respiratory Diseases
Journal of Microbiology (2024)