Abstract
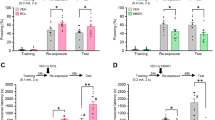
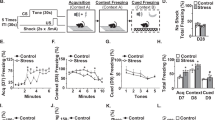
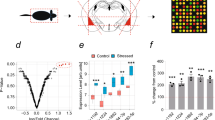
Disruption of persistent, stress-associated memories is relevant for treating posttraumatic stress disorder (PTSD) and related syndromes, which develop in a subset of individuals following a traumatic event. We previously developed a stress-enhanced fear learning (SEFL) paradigm in inbred mice that produces PTSD-like characteristics in a subset of mice, including persistently enhanced memory and heightened cFos in the basolateral amygdala complex (BLC) with retrieval of the remote (30-day-old) stress memory. Here, the contribution of BLC microRNAs (miRNAs) to stress-enhanced memory was investigated because of the molecular complexity they achieve through their ability to regulate multiple targets simultaneously. We performed small-RNA sequencing (smRNA-Seq) and quantitative proteomics on BLC tissue collected from mice 1 month after SEFL and identified persistently changed microRNAs, including mir-135b-5p, and proteins associated with PTSD-like heightened fear expression. Viral-mediated overexpression of mir-135b-5p in the BLC of stress-resilient animals enhanced remote fear memory expression and promoted spontaneous renewal 14 days after extinction. Conversely, inhibition of BLC mir-135b-5p in stress-susceptible animals had the opposite effect, promoting a resilient-like phenotype. mir-135b-5p is highly conserved across mammals and was detected in post mortem human amygdala, as well as human serum samples. The mir-135b passenger strand, mir-135b-3p, was significantly elevated in serum from PTSD military veterans, relative to combat-exposed control subjects. Thus, miR-135b-5p may be an important therapeutic target for dampening persistent, stress-enhanced memory and its passenger strand a potential biomarker for responsivity to a mir-135-based therapeutic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
28 June 2019
A Correction to this paper has been published: https://doi.org/10.1038/s41380-019-0452-y
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Arlington, VA, American Psychiatric Association, 2013.
Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27.
Schottenbauer MA, Glass CR, Arnkoff DB, Tendick V, Gray SH. Nonresponse and dropout rates in outcome studies on PTSD: review and methodological considerations. Psychiatry. 2008;71:134–68.
Sillivan SE, Joseph NF, Jamieson S, King ML, Chevere-Torres I, Fuentes I, et al. Susceptibility and resilience to posttraumatic stress disorder-like behaviors in inbred mice. Biol Psychiatry. 2017;82:924–33.
Levinsohn EA, Ross DA. To bend and not break: The neurobiology of stress, resilience, and recovery. Biol Psychiatry. 2017;82:e89–90.
Bredy TW, Lin Q, Wei W, Baker-Andresen D, Mattick JS. MicroRNA regulation of neural plasticity and memory. Neurobiol Learn Mem. 2011;96:89–94.
Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E. Deadenylation is a widespread effect of miRNA regulation. RNA. 2009;15:21–32.
Hou Q, Ruan H, Gilbert J, Wang G, Ma Q, Yao WD, et al. MicroRNA miR124 is required for the expression of homeostatic synaptic plasticity. Nat Commun. 2015;6:10045.
Lee K, Kim JH, Kwon OB, An K, Ryu J, Cho K, et al. An activity-regulated microRNA, miR-188, controls dendritic plasticity and synaptic transmission by downregulating neuropilin-2. J Neurosci. 2012;32:5678–87.
Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–9.
Fiorenza A, Lopez-Atalaya JP, Rovira V, Scandaglia M, Geijo-Barrientos E, Barco A. Blocking miRNA biogenesis in adult forebrain neurons enhances seizure susceptibility, fear memory, and food intake by increasing neuronal responsiveness. Cereb Cortex. 2016;26:1619–33.
Konopka W, Kiryk A, Novak M, Herwerth M, Parkitna JR, Wawrzyniak M, et al. MicroRNA loss enhances learning and memory in mice. J Neurosci. 2010;30:14835–42.
Dias BG, Goodman JV, Ahluwalia R, Easton AE, Andero R, Ressler KJ. Amygdala-dependent fear memory consolidation via miR-34a and Notch signaling. Neuron. 2014;83:906–18.
Volk N, Paul ED, Haramati S, Eitan C, Fields BK, Zwang R, et al. MicroRNA-19b associates with Ago2 in the amygdala following chronic stress and regulates the adrenergic receptor beta 1. J Neurosci. 2014;34:15070–82.
Griggs EM, Young EJ, Rumbaugh G, Miller CA. MicroRNA-182 regulates amygdala-dependent memory formation. J Neurosci. 2013;33:1734–40.
Lin Q, Wei W, Coelho CM, Li X, Baker-Andresen D, Dudley K, et al. The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat Neurosci. 2011;14:1115–7.
Mathew RS, Tatarakis A, Rudenko A, Johnson-Venkatesh EM, Yang YJ, Murphy EA, et al. A microRNA negative feedback loop downregulates vesicle transport and inhibits fear memory. eLife. 2016;5:e22467.
Wang RY, Phang RZ, Hsu PH, Wang WH, Huang HT, Liu IY. In vivo knockdown of hippocampal miR-132 expression impairs memory acquisition of trace fear conditioning. Hippocampus. 2013;23:625–33.
Schaefer A, O’Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, et al. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204:1553–8.
Young EJ, Blouin AM, Briggs SB, Sillivan SE, Lin L, Cameron MD, et al. Nonmuscle myosin IIB as a therapeutic target for the prevention of relapse to methamphetamine use. Mol Psychiatry. 2016;21:615–23.
Young EJ, Aceti M, Griggs EM, Fuchs RA, Zigmond Z, Rumbaugh G, et al. Selective, retrieval-independent disruption of methamphetamine-associated memory by actin depolymerization. Biol Psychiatry. 2014;75:96–104.
Rutten BPF, Vermetten E, Vinkers CH, Ursini G, Daskalakis NP, Pishva E, et al. Longitudinal analyses of the DNA methylome in deployed military servicemen identify susceptibility loci for post-traumatic stress disorder. Mol Psychiatry. 2018;23:1145–56.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Kasai A, Kakihara S, Miura H, Okada R, Hayata-Takano A, Hazama K, et al. Double in situ hybridization for microRNAs and mRNAs in brain tissues. Front Mol Neurosci. 2016;9:126.
Most D, Ferguson L, Blednov Y, Mayfield RD, Harris RA. The synaptoneurosome transcriptome: a model for profiling the emolecular effects of alcohol. Pharmacogenomics J. 2015;15:177–88.
Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–5.
Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, et al. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biol Psychiatry. 2017;81:1023–9.
Barez-Lopez S, Montero-Pedrazuela A, Bosch-Garcia D, Venero C, Guadano-Ferraz A. Increased anxiety and fear memory in adult mice lacking type 2 deiodinase. Psychoneuroendocrinology. 2017;84:51–60.
Montero-Pedrazuela A, Fernandez-Lamo I, Alieva M, Pereda-Perez I, Venero C, Guadano-Ferraz A. Adult-onset hypothyroidism enhances fear memory and upregulates mineralocorticoid and glucocorticoid receptors in the amygdala. PLoS ONE. 2011;6:e26582.
Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R, et al. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron. 2014;83:344–60.
Mannironi C, Biundo A, Rajendran S, De Vito F, Saba L, Caioli S, et al. miR-135a regulates synaptic transmission and anxiety-like behavior in amygdala. Mol Neurobiol. 2017;55:3301–15.
van Battum EY, Verhagen MG, Vangoor VR, Fujita Y, Derijck A, O’Duibhir E, et al. An Image-Based miRNA screen identifies miRNA-135s as regulators of CNS axon growth and regeneration by targeting Kruppel-like factor 4. J Neurosci. 2017;38:613–30.
Hu HY, Guo S, Xi J, Yan Z, Fu N, Zhang X, et al. MicroRNA expression and regulation in human, chimpanzee, and macaque brains. PLoS Genet. 2011;7:e1002327.
de Rie D, Abugessaisa I, Alam T, Arner E, Arner P, Ashoor H, et al. An integrated expression atlas of miRNAs and their promoters in human and mouse. Nat Biotechnol. 2017;35:872–8.
Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y. Long-lasting incubation of conditioned fear in rats. Biol Psychiatry. 2009;65:881–6.
Wu CaA P. MicroRNA passenger strand. Circulation Cardiovascular Genetics. 2014;7:567–8.
Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, et al. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202.
Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–7.
Andero R, Ressler KJ. Fear extinction and BDNF: translating animal models of PTSD to the clinic. Genes Brain Behav. 2012;11:503–12.
Velagapudi SP, Gallo SM, Disney MD. Sequence-based design of bioactive small molecules that target precursor microRNAs. Nat Chem Biol. 2014;10:291–7.
Stafford RL, Ear J, Knight MJ, Bowie JU. The molecular basis of the Caskin1 and Mint1 interaction with CASK. J Mol Biol. 2011;412:3–13.
Chornyy S, Parnis A, Shmoish M, Cassel D. High abundance of ArfGAP1 found in the mossy fibers in hilus of the dentate gyrus region of the mouse brain. PLoS ONE. 2017;12:e0189659.
Verheijen J, van der Zee J, Gijselinck I, Van den Bossche T, Dillen L, Heeman B, et al. Common and rare TBK1 variants in early-onset Alzheimer disease in a European cohort. Neurobiol Aging. 2018;62:245.e241–245.e247.
Acknowledgements
We thank the Scripps Florida Genomics Core for sequencing services, Nripesh Prasad at the Genomic Services Lab at Hudson Alpha for sequencing services and data analysis, Adrian Reich and the Bioinformatics Core for data analysis, the Mouse Behavior core and Alicia Brantley for assistance and behavioral equipment, all members of the Miller/Rumbaugh Labs for their technical assistance and thoughtful discussions. This work was funded by grants from the National Institute of Mental Health MH105400 and MH105400-02 (Diversity Supplement) (CM), National Institute of Neurological Disorders and Stroke NS096833 (CM), National Institute on Drug Abuse DA041469 (SS) and the Brain and Behavior Foundation-NARSAD Young Investigator Award (SS). This research project was supported in part by the Viral Vector Core of the Emory Neuroscience National Institute of Neurological Disorders and Stroke Core Facilities grant, P30NS055077. LdN and smRNA-Seq experiments in human serum were funded by a VIDI award number 91718336 from the Netherlands Scientific Organization (BR) and the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 707362 (LDN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sillivan, S.E., Jamieson, S., de Nijs, L. et al. MicroRNA regulation of persistent stress-enhanced memory. Mol Psychiatry 25, 965–976 (2020). https://doi.org/10.1038/s41380-019-0432-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0432-2
This article is cited by
-
Stress, microRNAs, and stress-related psychiatric disorders: an overview
Molecular Psychiatry (2023)
-
Orbitofrontal cortex microRNAs support long-lasting heroin seeking behavior in male rats
Translational Psychiatry (2023)
-
The stressed synapse 2.0: pathophysiological mechanisms in stress-related neuropsychiatric disorders
Nature Reviews Neuroscience (2022)
-
Targeting persistent stress-enhanced memory through microRNAs
Neuropsychopharmacology (2021)
-
Molecular Psychiatry, August 2020: new impact factor, and highlights of recent advances in psychiatry, including an overview of the brain’s response to stress during infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Molecular Psychiatry (2020)