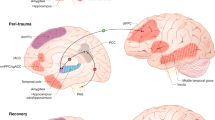
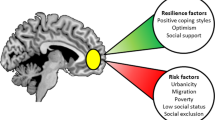
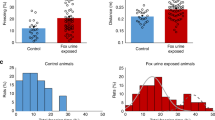
Abstract
Resilience is a neurobiological entity that shapes an individual’s response to trauma. Resilience has been implicated as the principal mediator in the development of mental illness following exposure to trauma. Although animal models have traditionally defined resilience as molecular and behavioral changes in stress responsive circuits following trauma, this concept needs to be further clarified for both research and clinical use. Here, we analyze the construct of resilience from a translational perspective and review optimal measurement methods and models. We also seek to distinguish between resilience, stress vulnerability, and posttraumatic growth. We propose that resilience can be quantified as a multifactorial determinant of physiological parameters, epigenetic modulators, and neurobiological candidate markers. This multifactorial definition can determine PTSD risk before and after trauma exposure. From this perspective, we propose the use of an ‘R Factor’ analogous to Spearman’s g factor for intelligence to denote these multifactorial determinants. In addition, we also propose a novel concept called ‘resilience reserve’, analogous to Stern’s cognitive reserve, to summarize the sum total of physiological processes that protect and compensate for the effect of trauma. We propose the development and application of challenge tasks to measure ‘resilience reserve’ and guide the assessment and monitoring of ‘R Factor’ as a biomarker for PTSD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–61.
Southwick SM, Bonanno GA, Masten AS, Panter-Brick C, Yehuda R. Resilience definitions, theory, and challenges: interdisciplinary perspectives. Eur J Psychotraumatol. 2014;5. https://doi.org/10.3402/ejpt.v5.25338.
Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–84.
Zannas AS, West AE. Epigenetics and the regulation of stress vulnerability and resilience. Neuroscience. 2014;264:157–70.
Juster RP, Bizik G, Picard M, Arsenault-Lapierre G, Sindi S, Trepanier L, et al. A transdisciplinary perspective of chronic stress in relation to psychopathology throughout life span development. Dev Psychopathol. 2011;23:725–76.
Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26:537–47.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60.
Harrison EL, Baune BT. Modulation of early stress-induced neurobiological changes: a review of behavioural and pharmacological interventions in animal models. Transl Psychiatry. 2014;4:e390.
Aburn G, Gott M, Hoare K. What is resilience? An integrative review of the empirical literature. J Adv Nurs. 2016;72:980–1000.
Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann NY Acad Sci. 2006;1071:379–96.
Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32.
Broekman BF. Stress, vulnerability and resilience, a developmental approach. Eur J Psychotraumatol. 2011;2. https://doi.org/10.3402/ejpt.v2i0.7229.
Meyerson DA, Grant KE, Carter JS, Kilmer RP. Posttraumatic growth among children and adolescents: a systematic review. Clin Psychol Rev. 2011;31:949–64.
Levine SZ, Laufer A, Hamama-Raz Y, Stein E, Solomon Z. Posttraumatic growth in adolescence: examining its components and relationship with PTSD. J Trauma Stress. 2008;21:492–6.
Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. J Trauma Stress. 1996;9:455–71.
Luthar SS, Cicchetti D, Becker B. The construct of resilience: a critical evaluation and guidelines for future work. Child Dev. 2000;71:543–62.
Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18:76–82.
Vogt D, Smith BN, King LA, King DW, Knight J, Vasterling JJ. Deployment risk and resilience inventory-2 (DRRI-2): an updated tool for assessing psychosocial risk and resilience factors among service members and veterans. J Trauma Stress. 2013;26:710–7.
Kobasa SC. Stressful life events, personality, and health: an inquiry into hardiness. J Pers Soc Psychol. 1979;37:1–11.
Rutter M. Resilience in the face of adversity. Protective factors and resistance to psychiatric disorder. Br J Psychiatry. 1985;147:598–611.
Lyons JA. Posttraumatic adjustment of Australian firefighters. J Nerv Ment Dis. 1989;177:248.
Green KT, Calhoun PS, Dennis MF, Mid-Atlantic Mental Illness Research, Education and Clinical Center Workgroup, Beckham JC. Exploration of the resilience construct in posttraumatic stress disorder severity and functional correlates in military combat veterans who have served since September 11, 2001. J Clin Psychiatry. 2010;71:823–30.
Davidson J, Baldwin DS, Stein DJ, Pedersen R, Ahmed S, Musgnung J, et al. Effects of venlafaxine extended release on resilience in posttraumatic stress disorder: an item analysis of the Connor-Davidson Resilience Scale. Int Clin Psychopharmacol. 2008;23:299–303.
Wrenn GL, Wingo AP, Moore R, Pelletier T, Gutman AR, Bradley B, et al. The effect of resilience on posttraumatic stress disorder in trauma-exposed inner-city primary care patients. J Natl Med Assoc. 2011;103:560–6.
Daniels JK, Hegadoren KM, Coupland NJ, Rowe BH, Densmore M, Neufeld RW, et al. Neural correlates and predictive power of trait resilience in an acutely traumatized sample: a pilot investigation. J Clin Psychiatry. 2012;73:327–32.
Wolf EJ, Miller MW, Sullivan DR, Amstadter AB, Mitchell KS, Goldberg J, et al. A classical twin study of PTSD symptoms and resilience: evidence for a single spectrum of vulnerability to traumatic stress. Depress Anxiety. 2018;35:132–9.
Pietrzak RH, Johnson DC, Goldstein MB, Malley JC, Rivers AJ, Morgan CA, et al. Psychosocial buffers of traumatic stress, depressive symptoms, and psychosocial difficulties in veterans of Operations Enduring Freedom and Iraqi Freedom: the role of resilience, unit support, and postdeployment social support. J Spec Oper Med. 2009;9:74–8.
Green KT, Hayward LC, Williams AM, Dennis PA, Bryan BC, Taber KH, et al. Examining the factor structure of the Connor-Davidson Resilience Scale (CD-RISC) in a post-9/11 U.S. military veteran sample. Assessment. 2014;21:443–51.
Vaishnavi S, Connor K, Davidson JR. An abbreviated version of the Connor-Davidson Resilience Scale (CD-RISC), the CD-RISC2: psychometric properties and applications in psychopharmacological trials. Psychiatry Res. 2007;152:293–7.
Campbell-Sills L, Stein MB. Psychometric analysis and refinement of the Connor-davidson Resilience Scale (CD-RISC): validation of a 10-item measure of resilience. J Trauma Stress. 2007;20:1019–28.
Maoz H, Goldwin Y, Lewis YD, Bloch Y. Exploring Reliability and Validity of the Deployment Risk and Resilience Inventory-2 among a nonclinical sample of discharged soldiers following mandatory military service. J Trauma Stress. 2016;29:556–62.
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9.
Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014;17:667–9.
Fernandes BS, Williams LM, Steiner J, Leboyer M, Carvalho AF, Berk M. The new field of ‘precision psychiatry’. BMC Med. 2017;15:80.
Janssen RJ, Mourao-Miranda J, Schnack HG. Making individual prognoses in psychiatry using neuroimaging and machine learning. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:798–808.
Bzdok D, Meyer-Lindenberg A. Machine learning for precision psychiatry: opportunities and challenges. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:223–30.
Horn SR, Charney DS, Feder A. Understanding resilience: new approaches for preventing and treating PTSD. Exp Neurol. 2016;284(Pt B):119–32.
Horn SR, Feder A. Understanding resilience and preventing and treating PTSD. Harv Rev Psychiatry. 2018;26:158–74.
Wu G, Feder A, Cohen H, Kim JJ, Calderon S, Charney DS, et al. Understanding resilience. Front Behav Neurosci. 2013;7:10.
Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–57.
Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene-stress-epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology. 2016;41:261–74.
Pinna G. In a mouse model relevant for post-traumatic stress disorder, selective brain steroidogenic stimulants (SBSS) improve behavioral deficits by normalizing allopregnanolone biosynthesis. Behav Pharmacol. 2010;21:438–50.
Devall AJ, Santos JM, Fry JP, Honour JW, Brandao ML, Lovick TA. Elevation of brain allopregnanolone rather than 5-HT release by short term, low dose fluoxetine treatment prevents the estrous cycle-linked increase in stress sensitivity in female rats. Eur Neuropsychopharmacol. 2015;25:113–23.
van der Werff SJ, Pannekoek JN, Stein DJ, van der Wee NJ. Neuroimaging of resilience to stress: current state of affairs. Hum Psychopharmacol. 2013;28:529–32.
Stillman AN, Aupperle RL. Neuroanatomical correlates of PTSD: risk, resiliency, and sequelae. In: Martin CR, Preedy VR, Patel VB, editors. Comprehensive guide to post-traumatic stress disorder. Cham: Springer International Publishing; 2014. p. 1–14.
Yang C, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci Rep. 2017;7:45942.
Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70.
Wells PS, Hirsh J, Anderson DR, Lensing AW, Foster G, Kearon C, et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet. 1995;345:1326–30.
Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, et al. An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry. 2016;173:980–8.
Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–12.
Jensen AR. Spearman’s g: links between psychometrics and biology. Ann N Y Acad Sci. 1993;702:103–29.
Loucks L, Yasinski C, Norrholm SD, Maples-Keller J, Post L, Zwiebach L, et al. You can do that?!: Feasibility of virtual reality exposure therapy in the treatment of PTSD due to military sexual trauma. J Anxiety Disord. 2018;61:55–63.
Rizzo A, Buckwalter JG, John B, Newman B, Parsons T, Kenny P, et al. STRIVE: stress resilience in virtual environments: a pre-deployment VR system for training emotional coping skills and assessing chronic and acute stress responses. Stud Health Technol Inform. 2012;173:379–85.
John BS, Oliva LS, Buckwalter JG, Kwok D, Rizzo AS. Self-reported differences in personality, emotion control, and presence between pre-military and non-military groups in a pilot study using the Stress Resilience in Virtual Environments (STRIVE) system. Stud Health Technol Inform. 2014;196:182–4.
Masten AS, Barnes AJ. Resilience in children: developmental perspectives. Children. 2018;5:98.
George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson-Jones BJ, Ducore J, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med. 2017;377:2215–27.
Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54.
McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8.
Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem. 2008;15:39–45.
Covington HE 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–60.
Covington HE 3rd, Maze I, Vialou V, Nestler EJ. Antidepressant action of HDAC inhibition in the prefrontal cortex. Neuroscience. 2015;298:329–35.
Covington HE 3rd, Vialou VF, LaPlant Q, Ohnishi YN, Nestler EJ. Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neurosci Lett. 2011;493:122–6.
Fujita Y, Morinobu S, Takei S, Fuchikami M, Matsumoto T, Yamamoto S, et al. Vorinostat, a histone deacetylase inhibitor, facilitates fear extinction and enhances expression of the hippocampal NR2B-containing NMDA receptor gene. J Psychiatr Res. 2012;46:635–43.
Han A, Sung YB, Chung SY, Kwon MS. Possible additional antidepressant-like mechanism of sodium butyrate: targeting the hippocampus. Neuropharmacology. 2014;81:292–302.
Jochems J, Boulden J, Lee BG, Blendy JA, Jarpe M, Mazitschek R, et al. Antidepressant-like properties of novel HDAC6-selective inhibitors with improved brain bioavailability. Neuropsychopharmacology. 2014;39:389–400.
Jochems J, Teegarden SL, Chen Y, Boulden J, Challis C, Ben-Dor GA, et al. Enhancement of stress resilience through histone deacetylase 6-mediated regulation of glucocorticoid receptor chaperone dynamics. Biol Psychiatry. 2015;77:345–55.
Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci. 2007;121:1125–31.
Lin H, Geng X, Dang W, Wu B, Dai Z, Li Y, et al. Molecular mechanisms associated with the antidepressant effects of the class I histone deacetylase inhibitor MS-275 in the rat ventrolateral orbital cortex. Brain Res. 2012;1447:119–25.
Matsumoto Y, Morinobu S, Yamamoto S, Matsumoto T, Takei S, Fujita Y, et al. Vorinostat ameliorates impaired fear extinction possibly via the hippocampal NMDA-CaMKII pathway in an animal model of posttraumatic stress disorder. Psychopharmacology. 2013;229:51–62.
Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, Matrisciano F, et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci USA. 2013;110:4804–9.
Pizzimenti CL, Lattal KM. Epigenetics and memory: causes, consequences and treatments for post-traumatic stress disorder and addiction. Genes Brain Behav. 2015;14:73–84.
Schmauss C. An HDAC-dependent epigenetic mechanism that enhances the efficacy of the antidepressant drug fluoxetine. Sci Rep. 2015;5:8171.
Stafford JM, Raybuck JD, Ryabinin AE, Lattal KM. Increasing histone acetylation in the hippocampus-infralimbic network enhances fear extinction. Biol Psychiatry. 2012;72:25–33.
Wagner FF, Zhang YL, Fass DM, Joseph N, Gale JP, Weiwer M, et al. Kinetically selective inhibitors of Histone Deacetylase 2 (HDAC2) as cognition enhancers. Chem Sci. 2015;6:804–15.
Whittle N, Schmuckermair C, Gunduz Cinar O, Hauschild M, Ferraguti F, Holmes A, et al. Deep brain stimulation, histone deacetylase inhibitors and glutamatergic drugs rescue resistance to fear extinction in a genetic mouse model. Neuropharmacology. 2013;64:414–23.
Yamawaki Y, Fuchikami M, Morinobu S, Segawa M, Matsumoto T, Yamawaki S. Antidepressant-like effect of sodium butyrate (HDAC inhibitor) and its molecular mechanism of action in the rat hippocampus. World J Biol Psychiatry. 2012;13:458–67.
Kirtley A, Thomas KL. The exclusive induction of extinction is gated by BDNF. Learn Mem. 2010;17:612–9.
Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013;18:813–23.
Moriguchi S, Shinoda Y, Yamamoto Y, Sasaki Y, Miyajima K, Tagashira H, et al. Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice. PLoS ONE. 2013;8:e60863.
Moriguchi S, Yamamoto Y, Ikuno T, Fukunaga K. Sigma-1 receptor stimulation by dehydroepiandrosterone ameliorates cognitive impairment through activation of CaM kinase II, protein kinase C and extracellular signal-regulated kinase in olfactory bulbectomized mice. J Neurochem. 2011;117:879–91.
Milanak ME, Judah MR, Berenbaum H, Kramer AF, Neider M. PTSD symptoms and overt attention to contextualized emotional faces: evidence from eye tracking. Psychiatry Res. 2018;269:408–13.
Graur S, Siegle G. Pupillary motility: bringing neuroscience to the psychiatry clinic of the future. Curr Neurol Neurosci Rep. 2013;13:365.
Zukerman G, Fostick L, Ben-Itzchak E. Early automatic hyperarousal in response to neutral novel auditory stimuli among trauma-exposed individuals with and without PTSD: an ERP study. Psychophysiology. 2018;55:e13217.
Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–28.
Daskalakis NP, Yehuda R, Diamond DM. Animal models in translational studies of PTSD. Psychoneuroendocrinology. 2013;38:1895–911.
Koolhaas JM, de Boer SF, Buwalda B, Meerlo P. Social stress models in rodents: towards enhanced validity. Neurobiol Stress. 2017;6:104–12.
Whitaker AM, Gilpin NW, Edwards S. Animal models of post-traumatic stress disorder and recent neurobiological insights. Behav Pharmacol. 2014;25:398–409.
Liberzon I, Knox D. Expanding our understanding of neurobiological mechanisms of resilience by using animal models. Neuropsychopharmacology. 2012;37:317–8.
Anacker C, Scholz J, O’Donnell KJ, Allemang-Grand R, Diorio J, Bagot RC, et al. Neuroanatomic differences associated with stress susceptibility and resilience. Biol Psychiatry. 2016;79:840–9.
Golden SA, Covington HE 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91.
Vasconcelos M, Stein DJ, de Almeida RM. Social defeat protocol and relevant biomarkers, implications for stress response physiology, drug abuse, mood disorders and individual stress vulnerability: a systematic review of the last decade. Trends Psychiatry Psychother. 2015;37:51–66.
Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–8.
Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J, et al. Association between posttraumatic stress disorder and inflammation: a twin study. Brain Behav Immun. 2013;30:125–32.
Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40:79–88.
Matsumoto K, Puia G, Dong E, Pinna G. GABA(A) receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress. 2007;10:3–12.
Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol. 2009;30:65–91.
Rothbaum BO, Kearns MC, Reiser E, Davis JS, Kerley KA, Rothbaum AO, et al. Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. J Clin Psychiatry. 2014;75:1380–7.
Agerbo E, Sullivan PF, Vilhjalmsson BJ, Pedersen CB, Mors O, Borglum AD, et al. Polygenic risk score, parental socioeconomic status, family history of psychiatric disorders, and the risk for schizophrenia: a Danish population-based study and meta-analysis. JAMA Psychiatry. 2015;72:635–41.
Middeldorp CM, Wray NR. The value of polygenic analyses in psychiatry. World Psychiatry. 2018;17:26–8.
Yehuda R, Flory JD, Bierer LM, Henn-Haase C, Lehrner A, Desarnaud F, et al. Lower methylation of glucocorticoid receptor gene promoter 1F in peripheral blood of veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:356–64.
Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–93.
Stark EA, Parsons CE, Van Hartevelt TJ, Charquero-Ballester M, McManners H, Ehlers A, et al. Post-traumatic stress influences the brain even in the absence of symptoms: A systematic, quantitative meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2015;56:207–21.
Sun D, Peverill MR, Swanson CS, McLaughlin KA, Morey RA. Structural covariance network centrality in maltreated youth with posttraumatic stress disorder. J Psychiatr Res. 2018;98:70–7.
Sun D, Davis SL, Haswell CC, Swanson CA, Mid-Atlantic MW, LaBar KS, et al. Brain structural covariance network topology in remitted posttraumatic stress disorder. Front Psychiatry. 2018;9:90.
Sun D, Haswell CC, Morey RA, De Bellis MD. Brain structural covariance network centrality in maltreated youth with PTSD and in maltreated youth resilient to PTSD. Dev Psychopathol. 2018:1–15. https://doi.org/10.1017/S0954579418000093.
Isingrini E, Perret L, Rainer Q, Amilhon B, Guma E, Tanti A, et al. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat Neurosci. 2016;19:560–3.
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8.
Zhu Z, Wang G, Ma K, Cui S, Wang JH. GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression. Oncotarget. 2017;8:35933–45.
Pfau ML, Purushothaman I, Feng J, Golden SA, Aleyasin H, Lorsch ZS, et al. Integrative analysis of sex-specific microRNA networks following stress in mouse nucleus accumbens. Front Mol Neurosci. 2016;9:144.
Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105.
Hoskins M, Pearce J, Bethell A, Dankova L, Barbui C, Tol WA, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206:93–100.
Kwapis JL, Wood MA. Epigenetic mechanisms in fear conditioning: implications for treating post-traumatic stress disorder. Trends Neurosci. 2014;37:706–20.
Litz BT. Early intervention for trauma: where are we and where do we need to go? A commentary. J Trauma Stress. 2008;21:503–6.
Litz B. Early intervention for trauma and loss: overview and working care model. Eur J Psychotraumatol. 2015;6:28543.
Rutter M. Annual research review: resilience–clinical implications. J Child Psychol Psychiatry. 2013;54:474–87.
Domhardt M, Munzer A, Fegert JM, Goldbeck L. Resilience in survivors of child sexual abuse: a systematic review of the literature. Trauma Violence Abus. 2015;16:476–93.
Legrand M, Troubat R, Brizard B, Le Guisquet AM, Belzung C, El-Hage W. Prefrontal cortex rTMS reverses behavioral impairments and differentially activates c-Fos in a mouse model of post-traumatic stress disorder. Brain Stimul. 2018;12:87–95.
Philip NS, Barredo J, van ‘t Wout-Frank M, Tyrka AR, Price LH, Carpenter LL. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 2018;83:263–72.
Carretta TR. U.S. Air Force pilot selection and training methods. Aviat Space Environ Med. 2000;71:950–6.
Mancini AD. Resilience and other reactions to military deployment: the complex task of identifying distinct adjustment trajectories. J Clin Psychiatry. 2014;75:e956–7.
Salum GA, Gadelha A, Pan PM, Moriyama TS, Graeff-Martins AS, Tamanaha AC, et al. High risk cohort study for psychiatric disorders in childhood: rationale, design, methods and preliminary results. Int J Methods Psychiatr Res. 2015;24:58–73.
Koch SBJ, Klumpers F, Zhang W, Hashemi MM, Kaldewaij R, van Ast VA, et al. The role of automatic defensive responses in the development of posttraumatic stress symptoms in police recruits: protocol of a prospective study. Eur J Psychotraumatol. 2017;8:1412226.
Baker DG, Nash WP, Litz BT, Geyer MA, Risbrough VB, Nievergelt CM, et al. Predictors of risk and resilience for posttraumatic stress disorder among ground combat Marines: methods of the Marine Resiliency Study. Prev Chronic Dis. 2012;9:E97.
Acknowledgements
We thank Emily K Clarke, Senior Clinical Research Specialist, Duke-UNC Brain Imaging and Analysis Center, Duke University School of Medicine for help with editing and proof-reading the manuscript. The authors report the following sources of support for their time working on this project: U.S. Department of Veterans Affairs grant IK2CX001397 (STS), I01CX001569 and I01CX001277 (CEM), I01CX000748 (RAM); National Institute of Mental Health (NIMH) grant R01MH111671 (RAM), National Institute of Neurological Disorders and Stroke (NINDS) R01NS086885 (RAM). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
STS has served on the advisory board for Jazz Pharmaceuticals, and as a consultant speaker for Neurocrine Biosciences, Teva Pharmaceutical Industries Ltd, and Otsuka/Lundbeck Pharmaceuticals. CEM is a co-applicant on pending patents focusing on neurosteroids in CNS disorders (no patents issued; no licensing in place; VA 208 waiver in place). All the remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rakesh, G., Morey, R.A., Zannas, A.S. et al. Resilience as a translational endpoint in the treatment of PTSD. Mol Psychiatry 24, 1268–1283 (2019). https://doi.org/10.1038/s41380-019-0383-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0383-7
This article is cited by
-
Bedtime regularity predicts positive affect among veterans with posttraumatic stress disorder: an ecological momentary assessment study
BMC Psychiatry (2023)
-
The Queensland Twin Adolescent Brain Project, a longitudinal study of adolescent brain development
Scientific Data (2023)
-
Posttraumatic stress symptom severity predicts cognitive decline beyond the effect of Alzheimer’s disease biomarkers in Veterans
Translational Psychiatry (2023)
-
Psychological Resilience in U.S. Military Veterans: Results from the 2019–2020 National Health and Resilience in Veterans Study
Psychiatric Quarterly (2023)
-
Rate and correlates of post-traumatic stress disorder (PTSD) following the Beirut blast and the economic crisis among Lebanese University students: a cross-sectional study
BMC Psychiatry (2022)