Abstract
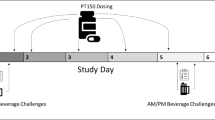
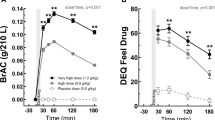
Rodent studies indicate that ghrelin receptor blockade reduces alcohol consumption. However, no ghrelin receptor blockers have been administered to heavy alcohol drinking individuals. Therefore, we evaluated the safety, tolerability, pharmacokinetic (PK), pharmacodynamic (PD) and behavioral effects of a novel ghrelin receptor inverse agonist, PF-5190457, when co-administered with alcohol. We tested the effects of PF-5190457 combined with alcohol on locomotor activity, loss-of-righting reflex (a measure of alcohol sedative actions), and on blood PF-5190457 concentrations in rats. Then, we performed a single-blind, placebo-controlled, within-subject human study with PF-5190457 (placebo/0 mg b.i.d., 50 mg b.i.d., 100 mg b.i.d.). Twelve heavy drinkers during three identical visits completed an alcohol administration session, subjective assessments, and an alcohol cue-reactivity procedure, and gave blood samples for PK/PD testing. In rats, PF-5190457 did not interact with the effects of alcohol on locomotor activity or loss-of-righting reflex. Alcohol did not affect blood PF-5190457 concentrations. In humans, all adverse events were mild or moderate and did not require discontinuation or dose reductions. Drug dose did not alter alcohol concentration or elimination, alcohol-induced stimulation or sedation, or mood during alcohol administration. Potential PD markers of PF-5190457 were acyl-to-total ghrelin ratio and insulin-like growth factor-1. PF-5190457 (100 mg b.i.d.) reduced alcohol craving during the cue-reactivity procedure. This study provides the first translational evidence of safety and tolerability of the ghrelin receptor inverse agonist PF-5190457 when co-administered with alcohol. PK/PD/behavioral findings support continued research of PF-5190457 as a potential pharmacological agent to treat alcohol use disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Edwards S, Kenna GA, Swift RM, Leggio L. Current and promising pharmacotherapies, and novel research target areas in the treatment of alcohol dependence: a review. Curr Pharm Des. 2011;17:1323–32.
Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311:1889–1900.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60.
Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. Ghrelin. Mol Metab. 2015;4:437–60.
Leggio L. Role of the ghrelin system in alcoholism: acting on the growth hormone secretagogue receptor to treat alcohol-related diseases. Drug News Perspect. 2010;23:157–66.
Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–7.
Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008;13:358–63.
Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, et al. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci USA. 2009;106:11318–23.
Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM, et al. Intravenous ghrelin administration increases alcohol craving in alcohol-dependent heavy drinkers: a preliminary investigation. Biol Psychiatry. 2014;76:734–41.
Farokhnia M, Grodin EN, Lee MR, Oot EN, Blackburn AN, et al. Exogenous ghrelin administration increases alcohol self-administration and modulates brain functional activity in heavy-drinking alcohol-dependent individuals. Mol Psychiatry. 2017. https://doi.org/10.1038/mp.2017.226.
Addolorato G, Capristo E, Leggio L, Ferrulli A, Abenavoli L, Malandrino N, et al. Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcohol Clin Exp Res. 2006;30:1933–7.
Badaoui A, De Saeger C, Duchemin J, Gihousse D, de Timary P, Starkel P. Alcohol dependence is associated with reduced plasma and fundic ghrelin levels. Eur J Clin Invest. 2008;38:397–403.
de Timary P, Cani PD, Duchemin J, Neyrinck AM, Gihousse D, Laterre PF, et al. The loss of metabolic control on alcohol drinking in heavy drinking alcohol-dependent subjects. PLoS ONE. 2012;7:e38682.
Leggio L, Ferrulli A, Cardone S, Nesci A, Miceli A, Malandrino N, et al. Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol. 2012;17:452–64.
Koopmann A, von der Goltz C, Grosshans M, Dinter C, Vitale M, Wiedemann K, et al. The association of the appetitive peptide acetylated ghrelin with alcohol craving in early abstinent alcohol dependent individuals. Psychoneuroendocrinology. 2012;37:980–6.
Kraus T, Schanze A, Groschl M, Bayerlein K, Hillemacher T, Reulbach U, et al. Ghrelin levels are increased in alcoholism. Alcohol Clin Exp Res. 2005;29:2154–7.
Suchankova P, Engel JA, Jerlhag E. Sub-chronic ghrelin receptor blockade attenuates alcohol- and amphetamine-induced locomotor stimulation in mice. Alcohol Alcohol. 2016;51:121–7.
Kaur S, Ryabinin AE. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin Exp Res. 2010;34:1525–34.
Gomez JL, Ryabinin AE. The effects of ghrelin antagonists [D-Lys(3)]-GHRP-6 or JMV2959 on ethanol, water, and food intake in C57BL/6J mice. Alcohol Clin Exp Res. 2014;38:2436–44.
Gomez JL, Cunningham CL, Finn DA, Young EA, Helpenstell LK, Schuette LM, et al. Differential effects of ghrelin antagonists on alcohol drinking and reinforcement in mouse and rat models of alcohol dependence. Neuropharmacology. 2015;97:182–93.
Stevenson JR, Buirkle JM, Buckley LE, Young KA, Albertini KM, Bohidar AE. GHS-R1A antagonism reduces alcohol but not sucrose preference in prairie voles. Physiol Behav. 2015;147:23–29.
Stevenson JR, Francomacaro LM, Bohidar AE, Young KA, Pesarchick BF, Buirkle JM, et al. Ghrelin receptor (GHS-R1A) antagonism alters preference for ethanol and sucrose in a concentration-dependent manner in prairie voles. Physiol Behav. 2016;155:231–6.
Landgren S, Simms JA, Hyytia P, Engel JA, Bartlett SE, Jerlhag E. Ghrelin receptor (GHS-R1A) antagonism suppresses both operant alcohol self-administration and high alcohol consumption in rats. Addict Biol. 2012;17:86–94.
Suchankova P, Steensland P, Fredriksson I, Engel JA, Jerlhag E. Ghrelin receptor (GHS-R1A) antagonism suppresses both alcohol consumption and the alcohol deprivation effect in rats following long-term voluntary alcohol consumption. PLoS ONE. 2013;8:e71284.
Chollet C, Meyer K, Beck-Sickinger AG. Ghrelin—a novel generation of anti-obesity drug: design, pharmacomodulation and biological activity of ghrelin analogues. J Pept Sci Off Publ Eur Pept Soc. 2009;15:711–30.
Els S, Beck-Sickinger AG, Chollet C. Ghrelin receptor: high constitutive activity and methods for developing inverse agonists. Methods Enzymol. 2010;485:103–21.
Pantel J, Legendre M, Cabrol S, Hilal L, Hajaji Y, Morisset S, et al. Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest. 2006;116:760–8.
Holst B, Schwartz TW. Ghrelin receptor mutations—too little height and too much hunger. J Clin Invest. 2006;116:637–41.
Bhattacharya SK, Andrews K, Beveridge R, Cameron KO, Chen C, Dunn M, et al. Discovery of PF-5190457, a potent, selective, and orally bioavailable ghrelin receptor inverse agonist clinical candidate. ACS Med Chem Lett. 2014;5:474–9.
Kong J, Chuddy J, Stock IA, Loria PM, Straub SV, Vage C, et al. Pharmacological characterization of the first in class clinical candidate PF-05190457: a selective ghrelin receptor competitive antagonist with inverse agonism that increases vagal afferent firing and glucose-dependent insulin secretion ex vivo. Br J Pharmacol. 2016;173:1452–64.
Denney WS, Sonnenberg GE, Carvajal-Gonzalez S, Tuthill T, Jackson VM. Pharmacokinetics and pharmacodynamics of PF-05190457: the first oral ghrelin receptor inverse agonist to be profiled in healthy subjects. Br J Clin Pharmacol. 2017;83:326–38.
Haass-Koffler CLAF, Swift RM, Leggio L. Altering ethanol pharmacokinetics to treat alcohol use disorder: can you teach an old dog new tricks?. J Psychopharmacol. 2017;31:812–8.
Kung DW, Coffey SB, Jones RM, Cabral S, Jiao W, Fichtner M, et al. Identification of spirocyclic piperidine-azetidine inverse agonists of the ghrelin receptor. Bioorg Med Chem Lett. 2012;22:4281–7.
Ghareeb M, Leggio L, El-Kattan A, Akhlaghi F. Development and validation of an UPLC-MS/MS assay for quantitative analysis of the ghrelin receptor inverse agonist PF-5190457 in human or rat plasma and rat brain. Anal Bioanal Chem. 2015;407:5603–13.
Cippitelli A, Karlsson C, Shaw JL, Thorsell A, Gehlert DR, Heilig M. Suppression of alcohol self-administration and reinstatement of alcohol seeking by melanin-concentrating hormone receptor 1 (MCH1-R) antagonism in Wistar rats. Psychopharmacol. 2010;211:367–75.
Farokhnia M, Schwandt ML, Lee MR, Bollinger JW, Farinelli LA, et al. Biobehavioral effects of baclofen in anxious alcohol-dependent individuals: a randomized, double-blind, placebo-controlled, laboratory study. Transl Psychiatry. 2017;7:e1108.
Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42:49–54.
MacLean AW, Fekken GC, Saskin P, Knowles JB. Psychometric evaluation of the Stanford Sleepiness Scale. J Sleep Res. 1992;1:35–39.
Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–6.
Fraser HF, Van Horn GD, Martin WR, Wolbach AB, Isbell H. Methods for evaluating addiction liability. (A) “Attitude” of opiate addicts toward opiate-like drugs. (B) a short-term “direct” addiction test. J Pharmacol Exp Ther. 1961;133:371–87.
McNair DM, Lorr M, Droppleman LF Manual for the Profile of Mood States. Educational and Industrial Testing Services: San Diego, CA, 1971.
Nijs IM, Franken IH, Muris P. The modified Trait and State Food-Cravings Questionnaires: development and validation of a general index of food craving. Appetite. 2007;49:38–46.
Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB. Naltrexone’s effects on reactivity to alcohol cues among alcoholic men. J Abnorm Psychol. 2000;109:738–42.
Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, et al. Naltrexone’s effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res. 1999;23:1386–94.
Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–6.
Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, et al. Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol. 1994;62:620–6.
Leggio L, Zywiak WH, McGeary JE, Edwards S, Fricchione SR, Shoaff JR, et al. A human laboratory pilot study with baclofen in alcoholic individuals. Pharmacol Biochem Behav. 2013;103:784–91.
Leggio L, Zywiak WH, Edwards SM, Tidey JW, Swift RM, Kenna GA. A preliminary double-blind, placebo-controlled randomized study of baclofen effects in alcoholic smokers. Psychopharmacol. 2015;232:233–43.
Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–97.
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. Second edn. SAS Institute Inc: Cary, NC, 2006.
Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31:310–39.
Cruz MT, Herman MA, Cote DM, Ryabinin AE, Roberto M. Ghrelin increases GABAergic transmission and interacts with ethanol actions in the rat central nucleus of the amygdala. Neuropsychopharmacology. 2013;38:364–75.
Ralevski E, Horvath TL, Shanabrough M, Hayden R, Newcomb J, Petrakis I. Ghrelin is supressed by intravenous alcohol and is related to stimulant and sedative effects of alcohol. Alcohol Alcohol. 2017;52:431–8.
Calissendorff J, Gustafsson T, Holst JJ, Brismar K, Rojdmark S. Alcohol intake and its effect on some appetite-regulating hormones in man: influence of gastroprotection with sucralfate. Endocr Res. 2012;37:154–62.
Calissendorff J, Danielsson O, Brismar K, Rojdmark S. Inhibitory effect of alcohol on ghrelin secretion in normal man. Eur J Endocrinol. 2005;152:743–7.
Calissendorff J, Danielsson O, Brismar K, Rojdmark S. Alcohol ingestion does not affect serum levels of peptide YY but decreases both total and octanoylated ghrelin levels in healthy subjects. Metabolism. 2006;55:1625–9.
Zimmermann US, Buchmann A, Steffin B, Dieterle C, Uhr M. Alcohol administration acutely inhibits ghrelin secretion in an experiment involving psychosocial stress. Addict Biol. 2007;12:17–21.
Tong J, Dave N, Mugundu GM, Davis HW, Gaylinn BD, Thorner MO, et al. The pharmacokinetics of acyl, des-acyl, and total ghrelin in healthy human subjects. Eur J Endocrinol. 2013;168:821–8.
Bang AS, Soule SG, Yandle TG, Richards AM, Pemberton CJ. Characterisation of proghrelin peptides in mammalian tissue and plasma. J Endocrinol. 2007;192:313–23.
Hosoda H, Kojima M, Mizushima T, Shimizu S, Kangawa K. Structural divergence of human ghrelin. Identification of multiple ghrelin-derived molecules produced by post-translational processing. J Biol Chem. 2003;278:64–70.
Howick K, Griffin BT, Cryan JF, Schellekens H. From Belly to Brain: targeting the ghrelin receptor in appetite and food intake regulation. Int J Mol Sci. 2017;18 pii: E273.
Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93:1980–7.
Takagi K, Legrand R, Asakawa A, Amitani H, Francois M, Tennoune N, et al. Anti-ghrelin immunoglobulins modulate ghrelin stability and its orexigenic effect in obese mice and humans. Nat Commun. 2013;4:2685.
Kuppens RJ, Diene G, Bakker NE, Molinas C, Faye S, Nicolino M, et al. Elevated ratio of acylated to unacylated ghrelin in children and young adults with Prader-Willi syndrome. Endocrine. 2015;50:633–42.
Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–5.
Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–8.
Anil Kumar P, Welsh GI, Saleem MA, Menon RK. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol. 2014;5:151.
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacol. 2003;168:3–20.
Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol. 2011;340:80–87.
Acknowledgements
This work was supported by NIH intramural funding ZIA-AA000218 (Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology; PI: Dr. Lorenzo Leggio), jointly supported by the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the Intramural Research Program of the National Institute on Drug Abuse (NIDA); and by the National Center for Advancing Translational Sciences (NCATS) grant UH2/UH3-TR000963 (PIs: Drs Lorenzo Leggio and Fatemeh Akhlaghi). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.The authors would like to thank the clinical and research staff involved in data collection and support at the NIAAA Division of Intramural Clinical and Biological Research, i.e., in the NIAAA/NIDA Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology (in particular Christian Frable, Allison Daurio and Sara Deschaine), in the Section on Molecular Pathophysiology (in particular Andrew Pilling and Caroline Rauffenbart) and in the NIAAA clinical intramural program. The authors would also like to thank the research staff involved in samples and data analysis in the Clinical Pharmacokinetics Research Laboratory at the University of Rhode Island (in particular Anitha Sravankumar, Ben Barlock and Sravani Adusumalli). The authors would also like to thank the clinical and research staff involved in data collection and patient care at the NIH Clinical Center, i.e., in the Department of Nursing (in particular the nurses of the 1SE Inpatient Unit and of the 1-HALC 1SE Outpatient Clinic), in the Department of Nutrition (in particular LT Kelly Ratteree, MPH, RDN and CDR Merel Kozlosky, MS, RD) and in the Department of Pharmacy. The authors would also like to thank Ms. Karen Smith and Ms. Holly Thompson from the NIH Library for bibliographic assistance. Furthermore, the authors would like to express their gratitude to the participants who took part in this study. Finally, the authors would like to thank the Steering Committee of the UH2/UH3-TR000963 grant (PIs: Drs Lorenzo Leggio and Fatemeh Akhlaghi) whose members included members from the NIAAA Division of Medication Development (in particular Dr. Joanne Fertig), the Drug Development Partnership Programs of the National Center for Advancing Translational Sciences (NCATS) and Pfizer, which kindly provided the study drug under the NCATS grant UH2/UH3-TR000963. Pfizer did not have any role in the study design, execution or interpretation of the results, and this publication does not necessarily represent the official views of Pfizer.
Author contributions
Study neuroscientific basis, rationale, concept and design: LL; Provided funding: LL, MH, and FA; Statistical analysis: MRL, JDT, MLS, and FA; Acquisition and management of data: MRL, JDT, MG, AAD, ANL, EC, LAF, SB, MF, MH, FA, and LL; Clinical and Safety monitoring: MRL, and LL; Administrative, technical, or material support: MRL, JDT, MG, MLS, AAD, ANL, EC, LAF, SB, MF, MH, FA, and LL; Analysis and interpretation of data: MRL, JDT, MG, MLS, AAD, ANL, EC, LAF, SB, MF, MH, FA, and LL; Drafting the manuscript: MRL, and LL. All authors have critically reviewed the manuscript for important intellectual content and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lee, M.R., Tapocik, J.D., Ghareeb, M. et al. The novel ghrelin receptor inverse agonist PF-5190457 administered with alcohol: preclinical safety experiments and a phase 1b human laboratory study. Mol Psychiatry 25, 461–475 (2020). https://doi.org/10.1038/s41380-018-0064-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0064-y
This article is cited by
-
Ghrelin decreases sensitivity to negative feedback and increases prediction-error related caudate activity in humans, a randomized controlled trial
Neuropsychopharmacology (2024)
-
Pharmacological Treatments for Alcohol Use Disorder: Considering the Role of Sex and Gender
Current Addiction Reports (2024)
-
Childhood sexual abuse is associated with higher total ghrelin serum levels in adulthood: results from a large, population-based study
Translational Psychiatry (2023)
-
Consideration of sex and gender differences in addiction medication response
Biology of Sex Differences (2022)
-
G-CuP: the effect of a forced oral glucose intake on alcohol craving and mesolimbic cue reactivity in alcohol dependence—study protocol of a randomized, double-blind, placebo-controlled crossover study
Trials (2022)