Abstract
We address some of the current limitations of translational research in fear memory and suggest alternatives that might help to overcome them. Appropriate fear responses are adaptive, but disruption of healthy fear memory circuits can lead to anxiety and fear-based disorders. Stress is one of the main environmental factors that can disrupt memory circuits and constitutes as a key factor in the etiopathology of these psychiatric conditions. Current therapies for anxiety and fear-based disorders have limited success rate, revealing a clear need for an improved understanding of their neurobiological basis. Although animal models are excellent for dissecting fear memory circuits and have driven tremendous advances in the field, translation of these findings into the clinic has been limited so far. Animal models of stress-induced pathological fear combined with powerful cutting-edge techniques would help to improve the translational value of preclinical studies. We also encourage combining animal and human research, including psychiatric patients in order to find new pharmacological targets with real therapeutic potential that will improve the extrapolation of the findings. Finally, we highlight novel neuroimaging approaches that improve our understanding of anxiety and fear-based disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–84.
Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–79.
Kanwar A, Malik S, Prokop LJ, Sim LA, Feldstein D, Wang Z, et al. The association between anxiety disorders and suicidal behaviors: a systematic review and meta-analysis. Depress Anxiety. 2013;30:917–29.
Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jönsson B. Group C study, et al. Econ Cost brain Disord Eur Eur J Neurol. 2012;19:155–62.
Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialog- Clin Neurosci. 2015;17:327–35.
Farb DH, Ratner MH. Targeting the modulation of neural circuitry for the treatment of anxiety disorders. Pharmacol Rev. 2014;66:1002–32.
Clark DM. Anxiety disorders: why they persist and how to treat them. Behav Res Ther. 1999;37:S5–27.
Kindt M. A behavioural neuroscience perspective on the aetiology and treatment of anxiety disorders. Behav Res Ther. 2014;62:24–36.
Wermter A-K, Laucht M, Schimmelmann BG, Banaschewski T, Banaschweski T, Sonuga-Barke EJS, et al.From nature versus nurture, via nature and nurture, to gene x environment interaction in mental disorders. Eur Child Adolesc Psychiatry. 2010;19:199–210.
Plumb TN, Cullen PK, Minor TR. Parameters of hormetic stress and resilience to trauma in rats. Stress. 2015;18:88–95.
Pêgo JM, Sousa JC, Almeida OFX, Sousa N. Stress and the neuroendocrinology of anxiety disorders. Curr Top Behav Neurosci. 2010;2:97–117.
McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29.
Roozendaal B, McEwen BS, Chattarji S, Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33.
Kendler KS. Genetic epidemiology in psychiatry. Taking both genes and environment seriously. Arch Gen Psychiatry. 1995;52:895–9.
Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–61.
Macphail EM. Cognitive function in mammals: the evolutionary perspective. Brain Res Cogn Brain Res. 1996;3:279–90.
Anderson DJ, Adolphs R. A framework for studying emotions across species. Cell. 2014;157:187–200.
Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35.
Lüthi A, Lüscher C. Pathological circuit function underlying addiction and anxiety disorders. Nat Neurosci. 2014;17:1635–43.
Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106–17.
Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35:320–30.
Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–5.
Brandão ML, Zanoveli JM, Ruiz-Martinez RC, Oliveira LC, Landeira-Fernandez J. Different patterns of freezing behavior organized in the periaqueductal gray of rats: Association with different types of anxiety. Behav Brain Res. 2008;188:1–13.
Santos M, D’Amico D, Spadoni O, Amador-Arjona A, Stork O, Dierssen M. Hippocampal hyperexcitability underlies enhanced fear memories in TgNTRK3, a panic disorder mouse model. J Neurosci. 2013;33:15259–71.
Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson R-M, Saksida LM, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–85.
Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, et al. Anxiety disorders. Nat Rev Dis Prim. 2017;3:17024
Sawamura T, Klengel T, Armario A, Jovanovic T, Norrholm SD, Ressler KJ, et al. Dexamethasone treatment leads to enhanced fear extinction and dynamic Fkbp5 regulation in amygdala. Neuropsychopharmacology. 2016;41:832–46.
Andero R, Dias BG, Ressler KJ. A role for Tac2, NkB, and Nk3 receptor in normal and dysregulated fear memory consolidation. Neuron. 2014;83:444–54.
Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–8.
Takahashi T, Morinobu S, Iwamoto Y, Yamawaki S. Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacol (Berl). 2006;189:165–73.
Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, et al. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–503.
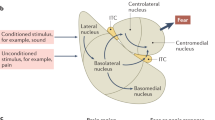
Maren S. Neurobiology of pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931.
Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–52.
Pape H-C, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–63.
Belzung C, Turiault M, Griebel G. Optogenetics to study the circuits of fear- and depression-like behaviors: a critical analysis. Pharmacol Biochem Behav. 2014;122:144–57.
Dejean C, Courtin J, Rozeske RR, Bonnet MC, Dousset V, Michelet T, et al. Neuronal circuits for fear expression and recovery: Recent advances and potential therapeutic strategies. Biol Psychiatry. 2015;78:298–306.
Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18:1213–25.
Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407.
LeDoux J. The amygdala. Curr Biol. 2007;17:R868–74.
Gafford GM, Ressler KJ. Mouse models of fear-related disorders: Cell-type-specific manipulations in amygdala. Neuroscience. 2016;321:108–20.
Sparta DR, Jennings JH, Ung RL, Stuber GD. Optogenetic strategies to investigate neural circuitry engaged by stress. Behav Brain Res. 2013;255:19–25.
Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–82.
Roth BL. DREADDs for neuroscientists. Neuron. 2016;89:683–94.
McCullough KM, Choi D, Guo J, Zimmerman K, Walton J, Rainnie DG, et al. Molecular characterization of Thy1 expressing fear-inhibiting neurons within the basolateral amygdala. Nat Commun. 2016;7:13149
Rask-Andersen M, Almén MS, Schiöth HB. Trends in the exploitation of novel drug targets. Nat Rev Drug Discov. 2011;10:579–90.
Zhu H, Roth BL. DREADD: a chemogenetic GPCR signaling platform. Int J Neuropsychopharmacol. 2014;18:pyu007.
Baratta MV, Kodandaramaiah SB, Monahan PE, Yao J, Weber MD, Lin P-A, et al. Stress enables reinforcement-elicited serotonergic consolidation of fear memory. Biol Psychiatry. 2016;79:814–22.
Meyer RM, Burgos-Robles A, Liu E, Correia SS, Goosens KA. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry. 2014;19:1284–94.
Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: Genetic and environmental influences on development of the stress response. Depress Anxiety. 2009;26:984–92.
Sharma S, Powers A, Bradley B, Ressler KJ. Gene × environment determinants of stress- and anxiety-related disorders. Annu Rev Psychol. 2016;67:239–61.
Yin H, Kauffman KJ, Anderson DG. Delivery technologies for genome editing. Nat Rev Drug Discov. 2017;16:387–99. https://doi.org/10.1038/nrd.2016.280.
Walters BJ, Azam AB, Gillon CJ, Josselyn SA, Zovkic IB. Advanced in vivo use of CRISPR/Cas9 and anti-sense DNA inhibition for gene manipulation in the brain. Front Genet. 2016;6:362.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science.2013;339:819–23.
Lee HB, Sundberg BN, Sigafoos AN, Clark KJ. Genome engineering with TALE and CRISPR systems in neuroscience. Front Genet. 2016;7:47.
Sheerin CM, Lind MJ, Bountress KE, Nugent NR, Amstadter AB. The genetics and epigenetics of PTSD: overview, recent advances, and future directions. Curr Opin Psychol. 2017;14:5–11.
Smoller JW. The genetics of stress-related disorders: PTSD, depression, and anxiety disorders. Neuropsychopharmacology. 2016;41:297–319.
Hamel EJO, Grewe BF, Parker JG, Schnitzer MJ. Cellular level brain imaging in behaving mammals: An engineering approach. Neuron. 2015;86:140–59.
Buzsáki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7:446–51.
Grewe BF, Helmchen F. Optical probing of neuronal ensemble activity. Curr Opin Neurobiol. 2009;19:520–9.
Grewe BF, Gründemann J, Kitch LJ, Lecoq JA, Parker JG, Marshall JD, et al. Neural ensemble dynamics underlying a long-term associative memory. Nature. 2017;543:670–5.
Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold K-H, Haass C, et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science. 2008;321:1686–9.
Reznichenko L, Cheng Q, Nizar K, Gratiy SL, Saisan PA, Rockenstein EM, et al. In vivo alterations in calcium buffering capacity in transgenic mouse model of synucleinopathy. J Neurosci. 2012;32:9992–8.
Walker DL, Ressler KJ, Lu K-T, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–51.
Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008;33:2108–16.
Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, et al. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am J Psychiatry. 2014;171:640–8.
de Kleine RA, Hendriks G-J, Kusters WJC, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71:962–8.
Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–44.
Mataix-Cols D, Fernández de la Cruz L, Monzani B, Rosenfield D, Andersson E, Pérez-Vigil A, et al. D-cycloserine augmentation of exposure-based cognitive behavior therapy for anxiety, obsessive-compulsive, and posttraumatic stress disorders: A systematic review and meta-analysis. JAMA Psychiatry. 2017;74:501–10. https://doi.org/10.1001/jamapsychiatry.2016.3955.
Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M, et al. Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Sci Transl Med. 2013;5:188ra73
Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013;18:813–23.
Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–6.
Bach DR, Tzovara A, Vunder J. Blocking human fear memory with the matrix metalloproteinase inhibitor doxycycline. Mol Psychiatry. 2017. https://doi.org/10.1038/mp.2017.65.
Brown TE, Wilson AR, Cocking DL, Sorg BA. Inhibition of matrix metalloproteinase activity disrupts reconsolidation but not consolidation of a fear memory. Neurobiol Learn Mem. 2009;91:66–72.
Arloth J, Bogdan R, Weber P, Frishman G, Menke A, Wagner KV, et al. Genetic differences in the immediate transcriptome response to stress predict risk-related brain function and psychiatric disorders. Neuron. 2015;86:1189–202.
Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, et al. Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci Biobehav Rev. 2017;77:247–85. https://doi.org/10.1016/j.neubiorev.2017.02.026.
Korn CW, Staib M, Tzovara A, Castegnetti G, Bach DR. A pupil size response model to assess fear learning. Psychophysiology. 2017;54:330–43.
Leuchs L, Schneider M, Czisch M, Spoormaker VI. Neural correlates of pupil dilation during human fear learning. Neuroimage. 2017;147:186–97.
McGinley MJ, David SV, McCormick DA. Cortical membrane potential signature of optimal states for sensory signal detection. Neuron. 2015;87:179–92.
Amadi U, Lim SH, Liu E, Baratta MV, Goosens KA. Hippocampal Processing of ambiguity enhances fear memory. Psychol Sci. 2017;28:143–61.
Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, et al. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–66.
Belova MA, Paton JJ, Morrison SE, Salzman CD, Braus DF, Buchel C, et al. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–84.
Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry. 2008;165:898–904.
Holaway RM, Heimberg RG, Coles ME. A comparison of intolerance of uncertainty in analogue obsessive-compulsive disorder and generalized anxiety disorder. J Anxiety Disord. 2006;20:158–74.
Dugas MJ, Gagnon F, Ladouceur R, Freeston MH. Generalized anxiety disorder: a preliminary test of a conceptual model. Behav Res Ther. 1998;36:215–26.
LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45.
Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: A systematic review. PLoS ONE. 2009;4:e5865
Quirici MB, da Rocha AJ. Teaching neuroimages: lipoid proteinosis (Urbach-Wiethe disease): typical findings in this rare genodermatosis. Neurology. 2013;80:e93.
Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–8.
Feinstein JS, Adolphs R, Damasio A, Tranel D, Naumann E, Bartussek D, et al. The human amygdala and the induction and experience of fear. Curr Biol. 2011;21:34–8.
Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Àvila-Parcet A, et al. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry. 2016;21:500–8.
Harrison B, Albajes-Eizagirre A, Soriano-Mas C, Vervliet B, Cardoner N, Benet O, et al. 14. Fear extinction in the human brain: Four meta-analyses of fMRI studies. Biol Psychiatry. 2017;81:S6–7.
Bach DR, Weiskopf N, Dolan RJ. A stable sparse fear memory trace in human amygdala. J Neurosci. 2011;31:9383–9.
Boubela RN, Kalcher K, Huf W, Seidel E-M, Derntl B, Pezawas L, et al. fMRI measurements of amygdala activation are confounded by stimulus correlated signal fluctuation in nearby veins draining distant brain regions. Sci Rep. 2015;5:10499
Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–24.
Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74:224–37.
Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin Psychol Rev. 2010;30:217–37.
Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88.
Picó-Pérez M, Radua J, Steward T, Menchón JM, Soriano-Mas C. Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:96–104.
Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends Cogn Sci. 2007;11:413–8.
Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry. 2013;70:1048–56.
Woo C-W, Chang LJ, Lindquist MA, Wager TD. Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci. 2017;20:365–77.
Koizumi A, Amano K, Cortese A, Shibata K, Yoshida W, Seymour B, et al. Fear reduction without fear through reinforcement of neural activity that bypasses conscious exposure. Nat Hum Behav. 2016;1:6
Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature. 2014;513:426–30.
Paunović N. Prolonged exposure counterconditioning as a treatment for chronic posttraumatic stress disorder. J Anxiety Disord. 2003;17:479–99.
Correia SS, McGrath AG, Lee A, Graybiel AM, Goosens KA. Amygdala-ventral striatum circuit activation decreases long-term fear. Elife. 2016; 5. https://doi.org/10.7554/eLife.12669.
Acknowledgements
RA is supported by a NARSAD Young Investigator Grant #22434, Ramón y Cajal program RYC2014-15784, RETOS-MINECO SAF2016-76565-R and FEDER funds. MAF is supported by a PERIS contract from the Departament de Salud of Generalitat de (SLT002/16/00490). CSM is supported by a Miguel Servet contract from the Carlos III Health Institute (CPII16/00048). MAF and CSM are supported by grants (PI16/00144 and PI16/00889) from the Carlos III Health Institute and FEDER funds -a way to build Europe-. AF is supported by a Juan de la Cierva contract from the Spanish government's Economy and Competitiveness Ministry (FJCI-2016-29888). We would like to thank Nicole Gouws for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RA declares intellectual property of the patent PCT/US2015/037629 “Methods of managing conditioned fear with neurokinin receptor antagonists”. The remaining authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Flores, Á., Fullana, M.À., Soriano-Mas, C. et al. Lost in translation: how to upgrade fear memory research. Mol Psychiatry 23, 2122–2132 (2018). https://doi.org/10.1038/s41380-017-0006-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-017-0006-0
This article is cited by
-
Refinement of the stress-enhanced fear learning model of post-traumatic stress disorder: a behavioral and molecular analysis
Lab Animal (2022)
-
Propranolol-induced inhibition of unconditioned stimulus-reactivated fear memory prevents the return of fear in humans
Translational Psychiatry (2020)
-
Revision to psychopharmacology mRNA and microRNA profiles are associated with stress susceptibility and resilience induced by psychological stress in the prefrontal cortex
Psychopharmacology (2020)
-
Rodent models of impaired fear extinction
Psychopharmacology (2019)