Abstract
Recent clinical trials have shown the promising therapeutic effects of pembrolizumab and nivolumab in patients with advanced gastric cancer. Currently, the programmed death ligand-1 (PD-L1) 22C3 pharmDx assay is the only companion diagnostic assay for assessing the safety and effectiveness of pembrolizumab. The purpose of this study was to compare 22C3 pharmDx and 28-8 pharmDx, a complementary diagnostic assay for nivolumab, in gastric cancer. In this study, 22C3 and 28-8 pharmDx assays were performed on the same formalin-fixed, paraffin-embedded tissue blocks of gastric adenocarcinoma clinical samples (n = 55). The concordance rate was evaluated using combined positive score (CPS) cutoffs of 1, 10, and 50. PD-L1 positivity with CPS ≥ 1 was 45.5% using the 22C3 pharmDx assay and 49.1% using the 28-8 pharmDx assay. At a CPS cutoff of 1, the overall percentage agreement was 96.4%. The positive and negative percentage agreements were 93.3% and 100%, respectively. All cases positive for PD-L1 using the 22C3 pharmDx assay were also positive using the 28-8 pharmDx assay. At a CPS cutoff of 10, the overall percentage agreement was 96.4%. At a CPS cutoff of 50, the two assays exhibited 100% concordance. Nonspecific cytoplasmic staining in the background tissues and tumor cells was often observed in the 28-8 pharmDx assay. When the results of the two assays were matched for response to immunotherapy, the overall response rate was higher in patients with a PD-L1 CPS ≥ 1 than in PD-L1-negative patients (22C3 pharmDx, P = 0.001; 28-8 pharmDx, P = 0.002). In conclusion, PD-L1 22C3 and 28-8 pharmDx assays were highly comparable at CPS cutoffs of 1, 10, and 50 in gastric cancer. These results provide evidence for the potential interchangeability of the two PD-L1 assays in gastric cancer.
Similar content being viewed by others
Introduction
Immune checkpoint inhibitors targeting the programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) pathway have become part of the standard of care in patients with several advanced cancers. PD-L1 expression, evaluated by immunohistochemistry (IHC), is a crucial biomarker for predicting the response to anti-PD-1/PD-L1 agents in several solid tumors [1]. Currently, four standardized PD-L1 IHC assays (22C3, 28-8, SP142, and SP263) have been developed specifically for predicting responses to pembrolizumab, nivolumab, atezolizumab, and durvalumab, respectively.
With respect to gastric cancer, the phase II KEYNOTE-059 trial demonstrated durable treatment responses to pembrolizumab in patients with recurrent locally advanced or metastatic tumors with PD-L1 positivity [2]. PD-L1 positivity was defined as a combined positive score (CPS) ≥ 1 using the Dako 22C3 pharmDx assay [2]. Based on this trial, the US Food and Drug Administration (FDA) granted accelerated approval for pembrolizumab in patients with advanced gastric cancer with a PD-L1 CPS ≥ 1, and for the 22C3 pharmDx assay as a companion diagnostic test [3]. In the following phase III KEYNOTE-061 trial, pembrolizumab failed to demonstrate significant improvement of overall survival as a second-line therapy for patients with advanced gastric cancer having a PD-L1 CPS ≥ 1 [4]. Nevertheless, patients with advanced gastric cancer and high levels of PD-L1 expression (CPS ≥ 10) achieved a significant therapeutic benefit [4], indicating that accurate evaluation of PD-L1 expression is still important. In a similar phase III study, nivolumab also demonstrated durable responses in refractory chemotherapy in patients with advanced gastric cancer [5, 6]. In a subgroup analysis, a survival benefit was shown, regardless of PD-L1 positivity [6]. However, only 40% of samples were assessed retrospectively for PD-L1 status, and only tumoral PD-L1 expression was analyzed [6]. Thus, the predictive significance of PD-L1 expression for nivolumab remains unclear and requires elucidation [7].
Currently, the 22C3 pharmDx assay is the only companion diagnostic assay for gastric cancer [3], and few studies have been conducted on the interchangeability of different PD-L1 assays for patients with gastric cancer [8, 9]. Each PD-L1 assay employs different antibodies using unique platforms, protocols, and cutoffs, which can be confusing to pathologists and clinicians. In addition, not all assays are available in all laboratories. More importantly, only a limited number of samples are available for certain patients. Therefore, there is an increasing need to harmonize PD-L1 assays in gastric cancer. In particular, the interchangeability of 22C3 and 28-8 pharmDx assays needs to be resolved, as the main clinical trials for gastric cancer involve the use of pembrolizumab and nivolumab. While high concordance rates between the 22C3 and 28-8 pharmDx assays have been reported in various solid tumors [10,11,12,13,14,15], there have been no comparative studies of the two assays in gastric cancer. This study aimed to directly compare the 22C3 and 28-8 pharmDx assays in gastric cancer clinical samples to evaluate the interchangeability of the two assays.
Materials and methods
Case selection and study design
The 22C3 pharmDx assay was performed for patients with gastric cancer (n = 55) as a clinical request at the Department of Pathology, Samsung Medical Center (Seoul, Korea) from May 2018 to October 2020. All patients were treated at the Samsung Medical Center between 1997 and 2020 and were at an advanced stage of the disease when the 22C3 pharmDx assay was performed. Of the 55 patients, four had stage I disease, and seven patients had stage II disease at the time of surgery (Table 1). However, local recurrence or distant metastasis developed during the follow-up period. For these patients, the PD-L1 test was performed using primary gastric tumor specimens. The 28-8 pharmDx assay was performed using formalin-fixed, paraffin-embedded blocks used for the 22C3 pharmDx assay. The PD-L1 IHC slides of the two assays were evaluated by two gastrointestinal pathologists (K-MK and SA) using a multi-head microscope, and the IHC slides from the 28-8 pharmDx assay were evaluated blinded to the 22C3 pharmDx results. Clinicopathological data such as age, sex, tumor location, histological type, Lauren classification, tumor stage according to the AJCC 8th edition staging manual, neoadjuvant therapy, and tissue type were evaluated by reviewing the medical records. The results of human epidermal growth factor receptor 2 (HER2) IHC, mismatch repair protein IHC, Epstein–Barr virus (EBV) in situ hybridization, and microsatellite instability (MSI) test were also retrieved from medical records; protocols for these assessments were described in a previous study [16]. HER2 IHC was performed for all patients. EBV status information was available for 53 patients, and MSI results were available for 39 patients.
For patients treated with anti-PD-1/PD-L1 agents, the treatment response was evaluated according to the RECIST criteria version 1.1 [17]. Patients with at least 6 weeks of follow-up period were included, and the response was assessed every 6–12 weeks according to iRECIST [18]. The primary outcome was the overall response rate (complete response plus partial response) and disease control rate (complete response, partial response plus stable disease) with respect to PD-L1 expression levels.
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB no. 2018-09-041-001) and all patients provided written informed consent before enrollment.
PD-L1 immunohistochemistry
Two PD-L1 pharmDx assays were performed for each case. Formalin-fixed, paraffin-embedded tissue sections (4 µm thick) were dried at 60 °C for 30 min. The pharmDx 22C3 assay (Dako, Carpinteria, CA) involved staining with an anti-PD-L1 22C3 mouse monoclonal primary antibody and the EnVision FLEX visualization system (Agilent, Santa Clara, CA) on an Autostainer Link 48 system (Dako), along with positive and negative controls, according to the manufacturer’s instructions. The 28-8 pharmDx assay was performed using an anti-PD-L1 28-8 rabbit monoclonal primary antibody on the same platform, according to the manufacturer’s instructions.
Scoring of PD-L1 assays
PD-L1 expression was evaluated in both tumors and immune cells. For tumor cells, positive PD-L1 staining was defined as complete and/or partial circumferential linear cellular membrane staining at any intensity [19, 20]. Tumor cells exhibiting only cytoplasmic staining were excluded [20]. Immune cells were scored as the proportion of tumor area covered with any discernible PD-L1 staining of any intensity in immune cells [20]. For immune cells, only tumor-associated lymphocytes and macrophages with convincing membrane and/or cytoplasmic staining were counted [20]. The CPS was calculated by dividing the number of PD-L1-stained cells (tumor cells and immune cells) by the total number of viable tumor cells, and multiplying the value by 10020. The maximum CPS value was set to 10020.
Evaluation of tumor-infiltrating lymphocytes
The degree of the host immune response was evaluated by a pathologist (K-MK) by reviewing hematoxylin and eosin slides. The edges of tumors were assessed for the presence of a prominent inflammatory reaction [16, 21]. When discrete lymphoid infiltrations were identified both within and around tumor cells, the tumor was defined as having high tumor-infiltrating lymphocytes (TILs) [16]. All other cases were defined as having low TILs [16].
Statistical analysis
Statistical analyses were performed using SPSS version 25.0 (SPSS, Chicago, IL). Contingency tables and χ2 tests were used to correlate PD-L1 IHC results with clinicopathological parameters and clinical outcomes. Cohen’s kappa coefficient of agreement was used to compare dichotomized expression values between assays. Kappa values below 0.50 were considered poor; values between 0.50 and 0.75 moderate; values between 0.75 and 0.90 good; and values above 0.90 excellent. Correlations between 22C3 and 28-8 pharmDx CPS were evaluated using Spearman’s rank correlation. Survival curves were drawn using Kaplan–Meier estimates and compared using log-rank tests. Statistical significance was set at P < 0.05. Finally, positive and negative percentage agreements were calculated.
Results
Patient characteristics and overall PD-L1 positivity
Patient demographics with respect to PD-L1 expression are shown in Table 1. The tissues were obtained by surgical resection from 49 (89.1%) patients and biopsy from 6 (10.9%) patients. All the cases were adenocarcinomas.
Using the 22C3 pharmDx assay, PD-L1 positivity with CPS ≥ 1 was identified in 25 (45.5%) of 55 patients. The PD-L1 CPS ranged from 0 to 95, with a mean CPS of 9.45. PD-L1 expression in tumor and immune cells was present in 10 (18.2%) and 25 (45.5%) patients, respectively. All patients with tumoral PD-L1 expression exhibited PD-L1 expression in immune cells. Higher levels of PD-L1 expression at a PD-L1 CPS ≥ 10 and CPS ≥ 50 were identified in 12 (21.8%) and 5 (9.1%) patients, respectively. PD-L1 expression (CPS ≥ 1) was significantly associated with older age (P = 0.001), male sex (P = 0.008), neoadjuvant chemotherapy (P = 0.037), and EBV positivity (P = 0.036) (Table 1). High PD-L1 expression (CPS ≥ 10) was significantly associated with older age (P = 0.008), male sex (P = 0.041), neoadjuvant chemotherapy (P = 0.029), high MSI status (P = 0.004), and high TIL infiltration (P = 0.005) (Supplementary Table).
Using the 28-8 pharmDx assay, PD-L1 expression with CPS ≥ 1 was detected in 27 (49.1%) of 55 patients. The PD-L1 CPS value ranged from 0 to 95, with a mean CPS of 10.71. Higher levels of PD-L1 expression at PD-L1 CPS ≥ 10 and CPS ≥ 50 were identified in 14 (25.5%) and 5 (9.1%) patients, respectively.
Comparison of 22C3 and 28-8 PD-L1 pharmDx assays
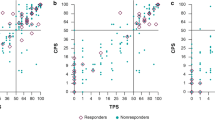
The comparison of PD-L1 expression detected using the 22C3 and 28-8 pharmDx assays is summarized in Table 2 and depicted in Fig. 1. At a CPS cutoff of 1, the overall percentage agreement was 96.36% (95% confidence interval [CI], 87.47–99.56%), and the concordance between the 22C3 and 28-8 assays was excellent (kappa value = 0.927). The positive percentage agreement was 93.33% (95% CI, 77.93–99.18%), and all patients who tested positive for PD-L1 in the 22C3 pharmDx assay also tested positive in the 28-8 pharmDx assay. The negative percentage agreement was 100% (95% CI, 86.28–100%). At a CPS cutoff of 10, the overall percentage agreement was 96.36% (95% CI, 87.47–99.56%), and the concordance between the 22C3 and 28-8 assays was excellent (kappa value = 0.899). The positive percentage agreement was 95.35% (95% CI, 84.19–99.43%), and the negative percentage agreement was 100% (95% CI, 73.54–100%). At a CPS cutoff of 50, the 22C3 and 28-8 assays displayed a 100% concordance rate.
Comparison of the quantitative CPS of the two assays showed a high correlation between the 22C3 and 28-8 pharmDx assays (Spearman correlation value = 0.978, P < 0.001). The CPS for the 28-8 and 22C3 assays was identical in 37 (67.3%) cases. The CPS of the 28-8 assay was higher than that of the 22C3 assay in 16 (29.1%) cases. Two (3.6%) cases showed a higher CPS in the 22C3 assay.
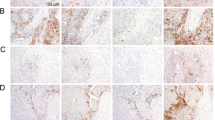
Representative figures of PD-L1 IHC using the two assays show comparable staining patterns with similar intensities (Fig. 2). Of the 55 tested cases, 2 (3.6%) were discordant at a CPS cutoff of 1. These two cases tested negative for PD-L1 in the 22C3 pharmDx assay and were PD-L1-positive (CPS = 1) in the 28-8 pharmDx assay. Surgically resected specimens were used in both the discordant cases. PD-L1 expression was detected in a few immune cells at the periphery of these tumors (Fig. 3). Compared to the 22C3 assay, the 28-8 assay often showed nonspecific staining (n = 29). Nonspecific staining in muscular or glandular tissue was identified in 26 cases, and nonspecific cytoplasmic staining in tumor cells was identified in 5 cases (Fig. 4). In contrast, the 22C3 pharmDx slides did not show nonspecific staining.
A Negative staining for PD-L1 in the 22C3 pharmDx assay. B Negative staining for PD-L1 in the 28-8 pharmDx assay. C A combined positive score of 70, as analyzed using the 22C3 pharmDx assay. D A combined positive score of 70, as analyzed using the 28-8 pharmDx assay. All images are at 2.5× magnification (insets at 20× magnification).
While no stained cells were observed in the 22C3 pharmDx assay (A), there were a few immune cells stained in the 28-8 pharmDx assay (arrow) (B). The combined positive score was measured as 1. The nonspecific cytoplasmic staining of tumor cells (arrowhead) was excluded from the scoring process (B). All images are at 10× magnification.
(A) Nonspecific background staining was not observed when the 28-8 pharmDx assay was used. (B) Nonspecific cytoplasmic staining in muscular tissue and tumor cells was observed when the 28-8 pharmDx assay was used. However, there was no membranous staining of tumor cells, and the staining was interpreted as negative staining. Images on the left are at 4× magnification; images on the right are at 20× magnification (of insets).
Treatment outcome
Between May 2018 and October 2020, 37 patients were treated with anti-PD-1/PD-L1 agents, and the treatment response was evaluated in 31 patients with a follow-up period of >6 weeks (pembrolizumab [n = 17], nivolumab [n = 12], and durvalumab [n = 2]). The median number of cycles for PD-1/PD-L1 agents was 8.9 (range, 1–37) as of January 20, 2021, and the median follow-up duration was 11.3 months.
The treatment responses of the patients are shown in Table 3. The overall response and disease control rates were 32.3% and 61.3%, respectively. The overall response rate differed according to PD-L1 status (64.3% [9/14] in the PD-L1-positive group [CPS ≥ 1] vs. 5.9% [1/17] in the PD-L1-negative group, P = 0.001) with FDA-approved 22C3 pharmDx. In addition, the disease control rate differed significantly between the two groups (85.7% [12/14] in the PD-L1-positive group vs. 41.2% [7/17] in the PD-L1-negative group, P = 0.025). The overall response rate was also significantly different at a CPS cutoff of 10 (63.6% [7/11] in PD-L1 CPS ≥ 10 vs. 15.0% [3/20] in PD-L1 CPS < 10, P = 0.013). The median overall survival after starting immunotherapy was 6.05 months (range, 2–20 months) in the PD-L1-negative group (CPS = 0) and 17.63 months (range 1–50 months) in the PD-L1-positive group (CPS ≥ 1). PD-L1 positivity was significantly associated with favorable overall survival (P = 0.033) (Fig. 5).
In the 28-8 pharmDx assay, the overall response rate also differed significantly according to the PD-L1 CPS 1 cutoff (P = 0.002). At a CPS cutoff of 10, the results of the overall response rate and disease control rate were the same in the 22C3 and 28-8 pharmDx assays.
Discussion
The PD-L1 22C3 pharmDx assay was approved by the US FDA as a companion diagnostic assay for the use of pembrolizumab in patients with advanced gastric cancer [3], and the PD-L1 CPS is considered a cost-effective screening tool that is easily used in clinics [22]. In the case of nivolumab, which also has promising results in advanced gastric cancer [5], there is currently no biomarker for predicting treatment responses. In addition, there are only a limited number of studies on PD-L1 as a biomarker. Therefore, PD-L1 diagnostic assays for the use of nivolumab are expected to be actively investigated to determine whether they can be used as complementary diagnostic assays in gastric cancer, as in other tumors such as lung cancer [23, 24]. We focused on directly comparing the detection of PD-L1 expression using 22C3 pharmDx, a companion diagnostic assay for pembrolizumab, and 28-8 pharmDx, a complementary diagnostic assay for nivolumab, in 55 patients with gastric cancer, to evaluate the interchangeability of the two assays.
PD-L1 assays for the use of pembrolizumab, nivolumab, and other PD-1/PD-L1 inhibitors have been developed independently, and they all require different methods. Therefore, questions have been raised as to whether the tests can be used interchangeably for treatment decisions for various PD-1/PD-L1 inhibitors [25]. Accordingly, several studies have evaluated the concordance of various PD-L1 assays in solid tumors, most prominently in lung cancers, with the 28-8 and 22C3 assays being two of the most frequently evaluated assays [10,11,12,13, 15]. According to the Blueprint project, in which the interchangeability of PD-L1 assays was evaluated in lung cancer, 22C3 and 28-8 assays were highly comparable and interchangeable for the evaluation of tumor cells [11], and comparable for the evaluation of immune cells [11]. In other studies comparing various PD-L1 assays in lung cancer, 22C3 and 28-8 demonstrated the highest concordance values for tumoral expression [12, 14]. It was also reported that the percentage of PD-L1 tumoral expression was slightly higher in the 28-8 assay compared to the 22C3 assay [12, 14]. Recently, Krigsfeld et al. reported a high concordance of tumoral PD-L1 expression between 22C3 and 28-8 pharmDx assays in >6000 clinical samples, mainly lung cancer and melanoma samples [10]. Overall, the percentage agreement was consistently >95% over a various range of PD-L1 tumoral cutoffs [10]. Meanwhile, there have been a few studies evaluating inter-assay variability of PD-L1 IHC in gastric cancer. Ma et al. compared SP142, 28-8, and E1L3N clones in gastric cancer and reported a high concordance between SP142 and 28-8 [9], and Park et al. reported a high concordance between 22C3 pharmDx and SP263 assays for CPS evaluation [8]. However, comparative studies of 22C3 and 28-8 pharmDx assays have not been previously conducted in gastric cancer.
In the present study, the results of 22C3 and 28-8 pharmDx assays were highly concordant, and the overall, positive, and negative percentage agreement between the two assays at a CPS cutoff of 1 were 96.36%, 93.33%, and 100%, respectively. In addition, the concordance rates at CPS cutoffs of 10 and 50 were 96.36% and 100%, respectively. Of the 55 tested cases, 2 (3.6%) cases were discordant at a CPS cutoff of 1 and were positive only in the 28-8 pharmDx assay. The CPS for these two cases was 1, and a few PD-L1-positive immune cells were identified at the periphery of these tumors. Of the two patients, one patient refractory to chemotherapy was enrolled in a clinical trial and treated twice with nivolumab. However, no treatment response was observed, and the patient died due to the progression of gastric cancer. The clinical significance of PD-L1 positivity for nivolumab remains unknown and needs to be investigated in a large gastric cancer cohort. In terms of quantitative comparison, the CPS of the 28-8 assay was higher than that of the 22C3 assay in 16 (29.1%) cases, whereas in two (3.6%) cases, a higher CPS was observed in the 22C3 assay. This result is in agreement with those of previous studies reporting a slightly higher percentage of PD-L1 expression detected by the 28-8 assay than by the 22C3 assay in lung cancer [12, 14].
Notably, nonspecific background staining was often observed in the 28-8 assay, in contrast to the 22C3 assay. In addition, nonspecific cytoplasmic staining was observed in tumor cells in five cases. Tumor cells exhibiting only cytoplasmic staining should not be included in CPS calculation [20]. Nonspecific staining can be caused by various factors such as antibodies, pre-analytic fixation, and processing of the specimen. Antibodies used in the 22C3 and 28-8 assays target the extracellular domain of the protein [26], and both assays use the same platform. The cause of nonspecific staining in the 28-8 pharmDx assay is uncertain, and we suggest that caution is required in the interpretation of the 28-8 pharmDx assay to exclude nonspecific staining. However, except for nonspecific staining, the two assays showed nearly identical staining patterns.
Interestingly, in the correlation of PD-L1 expression with clinicopathological factors, all four patients who received neoadjuvant chemotherapy showed PD-L1 expression. Elevation of PD-L1 expression after neoadjuvant chemotherapy has been reported in various types of solid tumors, including gastric cancer [27,28,29]. The mechanism underlying the role of chemotherapy in PD-L1 expression variation has not been elucidated completely in gastric cancer. Future studies on the predictive and prognostic value of PD-L1 expression in neoadjuvant settings are also required.
Our study had the limitation of a small sample size. However, to the best of our knowledge, this is the first study to compare 22C3 and 28-8 pharmDx assays in gastric cancer. In addition, this study was performed on clinical samples and not on tissue microarrays. When correlating the clinical outcome of immunotherapy with PD-L1 CPS using 22C3 and 28-8 pharmDx assays, both assays predicted the response with CPS 1 and 10 cutoffs. Despite the limitations of the small number of patients and insufficient follow-up period, our study adds to the existing evidence that the PD-L1 CPS is a clinically relevant biomarker that will improve the selection of patients who will best respond to immunotherapy in gastric cancer [30]. Notably, the results of both 22C3 pharmDx and 28-8 pharmDx assays correlated well with the clinical outcomes, suggesting that the 28-8 pharmDx assay can be used as a complementary test in gastric cancer.
In conclusion, we conducted a comparison of PD-L1 CPS between 22C3 pharmDx and 28-8 pharmDx assays in patients with gastric cancer and showed that the two assays are highly comparable at various CPS cutoffs. This study provides evidence for the potential interchangeability of these two assays in gastric cancer.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17:129.
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013.
Fashoyin-Aje L, Donoghue M, Chen H, He K, Veeraraghavan J, Goldberg KB, et al. FDA approval summary: pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist. 2019;24:103–9.
Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–33.
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–71.
Chen LT, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer. 2020;23:510–9.
Yamashita K, Iwatsuki M, Ajani JA, Baba H. Programmed death ligand-1 expression in gastrointestinal cancer: clinical significance and future challenges. Ann Gastroenterol Surg. 2020;4:369–78.
Park Y, Koh J, Na HY, Kwak Y, Lee KW, Ahn SH, et al. PD-L1 testing in gastric cancer by the combined positive score of the 22C3 PharmDx and SP263 assay with clinically relevant cut-offs. Cancer Res Treat. 2020;52:661–70.
Ma J, Li J, Qian M, Han W, Tian M, Li Z, et al. PD-L1 expression and the prognostic significance in gastric cancer: a retrospective comparison of three PD-L1 antibody clones (SP142, 28-8 and E1L3N). Diagn Pathol. 2018;13:91.
Krigsfeld GS, Prince EA, Pratt J, Chizhevsky V, William Ragheb J, Novotny J Jr., et al. Analysis of real-world PD-L1 IHC 28-8 and 22C3 pharmDx assay utilisation, turnaround times and analytical concordance across multiple tumour types. J Clin Pathol. 2020;73:656–64.
Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC, Botling J, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018;13:1302–11.
Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3:1051–8.
Shi L, Zhang SJ, Chen J, Lu SX, Fan XJ, Tong JH, et al. A comparability study of immunohistochemical assays for PD-L1 expression in hepatocellular carcinoma. Mod Pathol. 2019;32:1646–56.
Brunnstrom H, Johansson A, Westbom-Fremer S, Backman M, Djureinovic D, Patthey A, et al. PD-L1 immunohistochemistry in clinical diagnostics of lung cancer: inter-pathologist variability is higher than assay variability. Mod Pathol. 2017;30:1411–21.
Hendry S, Byrne DJ, Wright GM, Young RJ, Sturrock S, Cooper WA, et al. Comparison of four PD-L1 immunohistochemical assays in lung cancer. J Thorac Oncol. 2018;13:367–76.
Cho J, Ahn S, Son DS, Kim NK, Lee KW, Kim S, et al. Bridging genomics and phenomics of gastric carcinoma. Int J Cancer. 2019;145:2407–17.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52.
Phillips T, Simmons P, Inzunza HD, Cogswell J, Novotny J Jr., Taylor C, et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol. 2015;23:541–9.
PD-L1 IHC 22C3 pharmDx interpretation manual: gastric or gastroesophageal juction adenocarcinoma. Santa Clara: DAKO Agilent Technologies, 2018. https://www.agilent.com/cs/library/usermanuals/public/29219_pd-l1-ihc-22C3-pharmdx-gastric-interpretation-manual_us.pdf.
Hissong E, Ramrattan G, Zhang P, Zhou XK, Young G, Klimstra DS, et al. Gastric carcinomas with lymphoid stroma: an evaluation of the histopathologic and molecular features. Am J Surg Pathol. 2018;42:453–62.
Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–58.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39.
Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67.
Ionescu DN, Downes MR, Christofides A, Tsao MS. Harmonization of PD-L1 testing in oncology: a Canadian pathology perspective. Curr Oncol. 2018;25:e209–16.
Sunshine JC, Nguyen PL, Kaunitz GJ, Cottrell TR, Berry S, Esandrio J, et al. PD-L1 expression in melanoma: a quantitative immunohistochemical antibody comparison. Clin Cancer Res. 2017;23:4938–44.
Lee J, Kim ST, Kim K, Lee H, Kozarewa I, Mortimer PGS, et al. Tumor genomic profiling guides patients with metastatic gastric cancer to targeted treatment: The VIKTORY Umbrella Trial. Cancer Disco. 2019;9:1388–405.
Guo L, Song P, Xue X, Guo C, Han L, Fang Q, et al. Variation of programmed death ligand 1 expression after platinum-based neoadjuvant chemotherapy in lung cancer. J Immunother. 2019;42:215–20.
Mesnage SJL, Auguste A, Genestie C, Dunant A, Pain E, Drusch F, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC). Ann Oncol. 2017;28:651–7.
Wainberg ZA, Fuchs CS, Tabernero J, Shitara K, Muro K, Van Cutsem E, et al. Efficacy of pembrolizumab monotherapy for advanced gastric/gastroesophageal junction cancer with programmed death ligand 1 combined positive score ≥10. Clin Cancer Res. 2021;27:1923–31.
Funding
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2017R1E1A1A01075005) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR20C0025).
Author information
Authors and Affiliations
Contributions
SA and K-MK conceptualized and designed the study; SA and K-MK interpreted the immunohistochemistry slides, analyzed and interpreted the data, and performed statistical analysis; SA and K-MK wrote the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB no. 2018-09-041-001) and all patients provided written informed consent before enrollment.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ahn, S., Kim, KM. PD-L1 expression in gastric cancer: interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod Pathol 34, 1719–1727 (2021). https://doi.org/10.1038/s41379-021-00823-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00823-9
This article is cited by
-
Expression of PD-L1 clones (22C3 and 28-8) in hepatocellular carcinoma: a tertiary cancer care hospital experience
Egyptian Liver Journal (2024)
-
Current and Future Biomarkers in Esophagogastric Adenocarcinoma
Journal of Gastrointestinal Cancer (2024)
-
Scoring PD-L1 Expression in Urothelial Carcinoma: An International Multi-Institutional Study on Comparison of Manual and Artificial Intelligence Measurement Model (AIM-PD-L1) Pathology Assessments
Virchows Archiv (2024)
-
Expression of four cancer-testis antigens in TNBC indicating potential universal immunotherapeutic targets
Journal of Cancer Research and Clinical Oncology (2023)
-
Predictive biomarkers in gastric cancer
Journal of Cancer Research and Clinical Oncology (2023)