Abstract
Metaplastic thymomas are rare biphasic thymic tumors that are characteristically well-circumscribed, confined to the thymus, and follow a benign to indolent clinical course. Their relationship to other thymic neoplasms remains unclear, and their molecular characteristics have not been defined. We report for the first time recurrent translocation events in metaplastic thymomas involving the Yes Associated Protein 1 (YAP1) and Mastermind Like Transcriptional Coactivator 2 (MAML2) genes. Eight metaplastic thymomas were retrieved from two institutions’ archives over a 21-year period. Paraffin-embedded material from all cases underwent targeted DNA-based hybrid capture next-generation sequencing. Cases showing no somatic alterations subsequently underwent targeted RNA sequencing. Allele-specific real-time polymerase chain reaction was performed to detect GTF2I c.74146970T>A (p.L424H) mutations. All cases showed characteristic histologic features of metaplastic thymoma and demonstrated no local recurrence or distant metastatic disease at 1–22 years of follow-up. Six of eight cases were successfully sequenced, all showing YAP1-MAML2 fusions; in four cases the fusions were detected by DNA sequencing and in two cases by RNA sequencing. Two distinct products were identified: 5′ YAP1 exon 1 fused to 3′ MAML2 exons 2–5 or 5′ YAP1 exons 1–5 fused to 3′ MAML2 exons 2–5. All cases underwent allele-specific real-time polymerase chain reaction and demonstrated no GTF2I L424H mutations. Metaplastic thymoma is a distinct, clinically indolent thymic epithelial neoplasm characterized by YAP1-MAML2 fusion and lacking the GTF2I mutations found in Type A and AB thymomas.
Similar content being viewed by others
Introduction
Metaplastic thymoma is a rare biphasic tumor of the thymus typically found incidentally as a rounded anterior mediastinal mass in asymptomatic patients [1, 2], or in association with thymic cysts [3]. When originally described, this tumor was designated as, “thymoma with pseudosarcomatous stroma,” [1] or, by analogy with metaplastic carcinoma of the breast, “low-grade metaplastic carcinoma of the thymus [2].” The term “metaplastic thymoma” was adopted in the WHO classification of thymic tumors subsequent to 2004 [4].
Histologically, metaplastic thymoma is well-circumscribed or encapsulated, lacking the lobulation and fibrous bands of conventional thymomas and demonstrating a distinctive dual population of keratin-positive epithelioid cells in an anastomosing nested pattern and a variably cellular keratin-negative spindle cell component [1, 4,5,6]. The proportions of epithelioid and spindle cells vary between tumors, and some authors have proposed that the spindled cells represent a reactive stromal component based ontheir ultrastructural features [1], while others believe that both components are neoplastic based on their immunohistochemical and histologic features [2]. Metaplastic thymoma pursues a benign clinical course, with only rare reports of recurrence or malignant transformation.
The genomic alterations driving metaplastic thymoma have not previously been defined. In this study, we report for the first time recurrent translocation events involving Yes Associated Protein 1 (YAP1) and Mastermind Like Transcriptional Coactivator 2 (MAML2) genes in this tumor type.
Materials and methods
Patient selection and histologic evaluation
Paraffin-embedded formalin-fixed metaplastic thymoma tissue was obtained from hospital archives and authors’ consultation files following Brigham and Women’s Hospital Institutional Review Board approval. These included two cases from the Department of Pathology at Brigham and Women’s Hospital and six cases from the Department of Pathology, Queen Elizabeth Hospital, Hong Kong. Samples were acquired over a 21-year period. Demographic, clinical, and follow-up data were abstracted from the medical records and discussion with consulting pathologists.
At least one representative hematoxylin and eosin-stained whole tissue section was examined in each case. Tumors were diagnosed in accordance with the 2015 WHO classification [4].
Next-generation sequencing
Targeted next-generation sequencing including all exonic regions and selected introns of 447 cancer-associated genes was performed at Brigham and Women’s Hospital using OncoPanel (version 3). The target genes are listed in Supplementary Table 1. DNA was isolated from paraffin-embedded formalin-fixed tissue sections. Library preparation, custom hybrid capture (Agilent SureSelect, Agilent Technologies, Santa Clara, CA), and massively parallel sequencing (HiSeq 2500, Illumina, San Diego, CA) were performed as previously described [7, 8].
RNA sequencing
RNA fusion transcript detection was performed using Anchored Multiplex PCR. Total nucleic acid isolated from formalin-fixed paraffin-embedded tissue sections underwent reverse transcription, second strand synthesis, and adapter-ligation as previously described [9]. Sequencing libraries of targeted genes (Supplementary Table 1) were created using ArcherDx Fusion Plex Solid Tumor Kit (ArcherDx, Boulder, CO) and sequenced on an Illumina NextSeq 2. Fusion calling was performed using a laboratory-developed algorithm.
Thymic neoplasm data analysis
An institutional instance of cBioPortal [10] housed at the Dana Farber Cancer Institute containing genomic alterations from over 20,000 de-identified tumors including 52 thymic neoplasms was interrogated for the presence of YAP1-MAML2 rearrangements.
G2FT1A mutation analysis
DNA extracted from formalin-fixed paraffin-embedded tissues was subjected to allele-specific real-time polymerase chain reaction to detect GTF2I c.74146970T>A (p.L424H). PCR primer and probe sequences for detection of mutant (L424H) are as follows:
Forward primer GTF2I-F: 5′-GAT CCC GTA CCC TCT TTT CC-3′.
Reverse primer (mutant specific) GTF2I-R2: 5′-AAC GAA TCC TTT CCT TTG TATG-3′.
Taqman probe GTF2I-TP4: [FAM] 5′-TAT CCT CTC CAG GCG AGG AAT T-3′[MGB-NFQ].
For control PCR, the forward primer and probe are the same as for L424H PCR, while the reverse primer is as follows:
Reverse primer GTF2I-CR: 5′-CAG GAA TCC AAG AGT CTT ACT TC-3′.
Results
Demographic and clinicopathologic features are summarized in Table 1. Study patients consisted of one man and seven women with a median age of 55 (Q1-Q3: 43–59). The tumors ranged from 2.5 to 8.2 cm in size and presented as incidental imaging findings in five of seven patients. Two additional patients presented with chest pain and mild ocular myasthenia gravis, respectively. Clinical follow-up was available in six patients, all of whom were alive and well at 1–22 years after resection (median 8.25; Q1–Q3:3.75–9.75). No recurrences, local invasion, or distant metastases were noted in any of the patients.
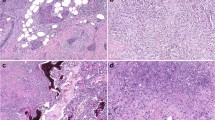
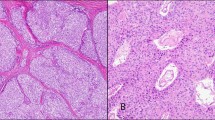
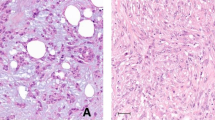
Histologic evaluation revealed circumscribed biphasic tumors consisting of variably sparse to abundant anastomosing nests of epithelioid tumor cells embedded in a cellular stroma characterized by delicately elongate bland-looking spindle cells (Fig. 1). Atypia, including nucleomegaly and hyperchromasia with a smudged chromatin pattern, was frequently present in the epithelioid component; however, mitotic figures were infrequent and necrosis was absent in all cases. Lymphocytes were sparse within the tumors. A compressed rim of normal thymic tissue could often be identified at the periphery.
a Gross image of a metaplastic thymoma with a firm tan-white cut surface and lobulated but well-circumscribed border. b Metaplastic thymomas are also microscopically well-circumscribed. This tumor demonstrated numerous irregular nests of epithelioid cells at the periphery of the tumor (×40), while c central areas showed fewer epithelioid nests and a more prominent spindle cell component with a storiform growth pattern (×100). d Metaplastic thymoma with more evenly distributed epithelioid component presenting in an interconnected trabecular pattern (×40), with e a sparse lymphocytic infiltrate in both stromal and epithelioid components (×200). f Nuclear atypia characterized by hyperchromasia, nucleomegaly, and irregular nuclear contours of the epithelioid component was seen in most tumors (×200)
Archival paraffin-embedded tissue was used for molecular analysis (Table 2). Next-generation sequencing failed in two cases due to poor nucleic acid quality, one each from 1997 and 2008. DNA-based targeted next-generation sequencing identified a rearrangement involving the YAP1 and MAML2 genes in four tumors (patients 1, 3, 5, and 8). The breakpoint locations (Table 2) occurred in intron 1/exon 2 (cases 3, 5, and 8; Supplementary Fig. 1) and intron 5 (case 1) of YAP1. In all four cases the MAML2 breakpoint mapped to intron 1. In two cases (2 and 4), sequencing was successful, but no fusions or somatic mutations were detected. Anchored multiplex PCR performed on these two cases identified YAP1-MAML2 transcripts fusing exon 5 of the YAP1 gene and exon 2 of the MAML2 gene resulting from an intrachromosomal inversion event. No other fusion events were detected. In all cases, predicted functional (in-frame) fusion events showed an orientation of 5′ YAP1 and 3′ MAML2. Copy number profiling by DNA sequencing showed one copy deletion of the C-terminus of YAP1 beginning at the predicted breakpoint in five of six cases, providing further support for a rearrangement event. Of note, C-terminus YAP1 gene deletion was evident by DNA sequencing in one case with a fusion detected only by RNA sequencing. No other recurrent alterations were identified in the cohort. Additional somatic variants were detected in only two cases, including monosomy of chromosomes 13 and 22 as well as truncating mutations in RSPO2 and MED12 (case 5); and gain of 5q and deletions on 11q and 18 (case 8).
The genomic profiles of 52 thymic neoplasms previously sequenced by OncoPanel at our institution as part of the PROFILE institutional sequencing initiative were examined for YAP1-MAML2 rearrangements [11]. The institutional cohort contained 25 thymomas, 25 thymic carcinomas, and 2 thymic neuroendocrine tumors. No fusion events were detected. Expected genomic alterations were observed in thymic carcinomas including TP53 mutations in nine (36%) cases and CDKN2A deletion in seven (28%) cases. No recurrent pathogenic or likely pathogenic variants were detected in the thymomas.
Allele-specific RT-PCR showed no GTF2I c.74146970T>A mutation in any of the tumors.
Discussion
Metaplastic thymomas are very rare, with only 30 cases reported in the English language literature (Table 3). This study represents the largest case series, with long follow-up information. Combining all available data, the tumor occurs predominantly in middle-aged subjects, with slight female predominance. In contrast to conventional thymomas (type A, AB, B1, B2, or B3), there is a low frequency of association with myasthenia gravis (3 of 38) [12, 13]. The clinical outcome also appears more favorable, with only one reported recurrence [2]. Although two cases have been reported to show histologic transformation to sarcomatoid carcinoma [14, 15], there is no follow-up information on the consequence of this phenomenon.
Metaplastic thymoma shows highly characteristic morphologic features distinct from conventional thymomas. Furthermore, intratumoral lymphoid cells are generally sparse and are TdT-negative [4], with the exception of one reported case with patchy infiltrates of TdT-positive lymphoid cells and accompanied by myasthenia gravis [12]. The histogenesis and relationship between metaplastic thymoma and other thymic epithelial neoplasms remain unknown. Based on the presence of keratin-negative, EMA-positive spindled cells in a subset of type AB thymomas, Miki et al. have suggested a relationship between metaplastic thymoma and type AB thymoma [16]. A previous study of seven cases demonstrated no gross chromosomal abnormalities by comparative genomic hybridization [17], but comprehensive studies of genetic alterations in metaplastic thymoma to clarify its relationship to other thymic tumors are lacking.
In this study, all six tumors successfully analyzed by sequencing methods demonstrated YAP1-MAML2 fusions, suggesting a common pathogenetic mechanism. Two distinct products were identified: 5′ YAP1 exon 1 fused to 3′ MAML2 exons 2–5 or 5′ YAP1 exons 1–5 fused to 3′ MAML2 exons 2–5.
MAML2 is a transcription coactivator that directly binds to notch proteins via its N-terminal basic domain and results in upregulation of notch pathway target genes [18]. Fusions of the MAML2 gene to the CRTC1 and CRTC3 genes are distinctive of mucoepidermoid carcinoma, but have also been described in ovarian cancer cell lines [19,20,21]. MLL-MAML2 translocations have been described in acute myelogenous leukemia and myelodysplastic syndrome [22]. In mucoepidermoid carcinoma, CRTC1-MAML2 fusions involving breakpoints in exons 2–5 of MAML2 result in a fusion product with a retained MAML2 transactivation domain [20]. Consequent activation of select notch pathway elements and cAMP/CREB-responsive genes may play a role in malignant transformation [19, 20], although other studies suggest that MAML2 translocations that do not include the N-terminal basic domain may activate genes unrelated to the notch pathway [18]. YAP1, a transcriptional coactivator regulated by the Hippo and Wnt pathways, plays a role in organ growth during normal development, and its overexpression in cancer results in transcriptional activation of proliferation-associated genes that is dependent on its N-terminal TEAD-binding domain [23]. YAP1 also plays a role in mechanotransduction of cell-extracellular matrix interactions and contact inhibition of cell growth in both cancer cells and cancer-associated fibroblasts [23].
YAP1-MAML2 rearrangements have recently been described in cell lines of ovarian carcinoma, glioblastoma, tongue squamous cell carcinoma, and rare cases of nasopharyngeal carcinoma. The gene fusion yields a fusion protein that acts via a TEAD1-dependent mechanism to generate a YAP1-associated transcriptional signature and thereby promote tumor cell growth [24]. Recently, RNA sequencing studies of poroma, a benign skin tumor, and porocarcinoma, its malignant counterpart, identified YAP1-MAML2 fusions in 71 of 104 (68%) poromas and 1 of 11 (9%) of porocarcinomas [25]. As in our study, a variant YAP1 breakpoint occurring in intron 1 was also detected in over 15% of poromas tested. The YAP1 TEAD-binding domain is encoded by exon 1 and therefore is conserved even when the breakpoint falls in intron 1. Sekine et al. confirmed that both the YAP1(e5)-MAML2 and YAP1(e1)-MAML2 fusions upregulate TEAD-dependent transcription in in vitro studies [25].
Review of our DNA panel sequencing coverage showed limited capture of intronic regions in introns 5 and 6 of YAP1; this likely explains the inability of OncoPanel to detect fusions in the two cases with confirmed fusions by RNA sequencing. The limited sensitivity of DNA-based sequencing for rearrangement events has been well-documented [26, 27]. The use of DNA-based sequencing is a limitation of our institutional analysis of thymic neoplasms and leaves open the possibility that similar fusions have gone undetected historically. However, YAP1-MAML2 fusions in thymomas or thymic carcinomas have also not been reported in the literature despite several major efforts to genomically characterize thymic neoplasms [28, 29].
GTF2I c.74146970T>A (p.L424H) mutation occurs at a high frequency in type A and type AB thymomas (79–87%) and a low frequency in type B thymomas (0–32%) [28,29,30]. The molecular findings of the specific YAP1-MAML2 fusions and lack of GTF2I L424H mutation provide a strong support that metaplastic thymoma is distinct from conventional thymomas (types A, AB, B1, B2, and B3). YAP1-MAML2 fusion likely represents a driver of neoplasia. Our findings therefore support classification of metaplastic thymoma separate from other thymomas, as an indolent tumor with excellent prognosis that can be diagnosed on morphologic grounds.
References
Suster S, Moran CA, Chan JK. Thymoma with pseudosarcomatous stroma: report of an unusual histologic variant of thymic epithelial neoplasm that may simulate carcinosarcoma. Am J Surg Pathol. 1997;21:1316–23.
Yoneda S, Marx A, Heimann S, Shirakusa T, Kikuchi M, Muller-Hermelink HK. Low-grade metaplastic carcinoma of the thymus. Histopathology. 1999;35:19–30.
Poorabdollah M, Mehdizadeh E, Mohammadi F, Sabeti S. Metaplastic thymoma: report of an unusual thymic epithelial neoplasm arising in the wall of a thymic cyst. Int J Surg Pathol. 2009;17:51–4.
Chen G, Chan JKC, Marchevsky AM, Marom EM, Marx A, et al. Metaplastic thymoma. In: Travis WD, BE, Burke AP, Marx A, Nicholson AG, editors. World health organization classification of tumours. pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon, France: IARC Press; 2015. p. 207–9.
Jin M, Liu B, Wang L, Xu JY. Clinicopathologic study of metaplastic thymoma. Zhonghua Bing Li Xue Za Zhi. 2006;35:285–8.
Noh TW, Kim SH, Lim BJ, Yang WI, Chung KY. Thymoma with pseudosarcomatous stroma. Yonsei Med J. 2001;42:571–5.
Sholl LM, Do K, Shivdasani P, Cerami E, Dubuc AM, Kuo FC, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight. 2016;1:e87062.
Garcia EP, Minkovsky A, Jia Y, Ducar MD, Shivdasani P, Gong X, et al. Validation of OncoPanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141:751–8.
Zheng Z, Liebers M, Zhelyazkova B, Cao Y, Panditi D, Lynch KD, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–84.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1.
MacConaill LE, Garcia E, Shivdasani P, Ducar M, Adusumilli R, Breneiser M, et al. Prospective enterprise-level molecular genotyping of a cohort of cancer patients. J Mol Diagn. 2014;16:660–72.
Tajima S, Yanagiya M, Sato M, Nakajima J, Fukayama M. Metaplastic thymoma with myasthenia gravis presumably caused by an accumulation of intratumoral immature T cells: a case report. Int J Clin Exp Pathol. 2015;8:15375–80.
Kang G, Yoon N, Han J, Kim YE, Kim TS, Kim K. Metaplastic thymoma: report of 4 cases. Korean J Pathol. 2012;46:92–5.
Moritani S, Ichihara S, Mukai K, Seki Y, Inoue S, Yasuda A, et al. Sarcomatoid carcinoma of the thymus arising in metaplastic thymoma. Histopathology. 2008;52:409–11.
Lu HS, Gan MF, Zhou T, Wang SZ. Sarcomatoid thymic carcinoma arising in metaplastic thymoma: a case report. Int J Surg Pathol. 2011;19:677–80.
Miki YHK, Yoshino T, Miyatani K, Takahashi K. Type AB thymoma is not a mixed tumor of type A and type B thymomas, but a distinct type of thymoma. Virchows Arch. 2014;464:725–34.
Liu B, Rao Q, Zhu Y, Yu B, Zhu HY, Zhou XJ. Metaplastic thymoma of the mediastinum. A clinicopathologic, immunohistochemical, and genetic analysis. Am J Clin Pathol. 2012;137:261–9.
Kitagawa M. Notch signalling in the nucleus: roles of Mastermind-like (MAML) transcriptional coactivators. J Biochem. 2016;159:287–94.
Coxon A, Rozenblum E, Park YS, Joshi N, Tsurutani J, Dennis PA, et al. Mect1-Maml2 fusion oncogene linked to the aberrant activation of cyclic AMP/CREB regulated genes. Cancer Res. 2005;65:7137–44.
Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, et al. t(11;19)(q21; p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33:208–13.
Fehr A, Roser K, Heidorn K, Hallas C, Loning T, Bullerdiek J. A new type of MAML2 fusion in mucoepidermoid carcinoma. Genes Chromosomes Cancer. 2008;47:203–6.
Nemoto N, Suzukawa K, Shimizu S, Shinagawa A, Takei N, Taki T, et al. Identification of a novel fusion gene MLL-MAML2 in secondary acute myelogenous leukemia and myelodysplastic syndrome with inv(11)(q21q23). Genes Chromosomes Cancer. 2007;46:813–9.
Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–312.
Picco G, Chen ED, Alonso LG, Behan FM, Goncalves E, Bignell G, et al. Functional linkage of gene fusions to cancer cell fitness assessed by pharmacological and CRISPR-Cas9 screening. Nat Commun. 2019;10:2198.
Sekine S, Kiyono T, Ryo E, Ogawa R, Wakai S, Ichikawa H, et al. Recurrent YAP1-MAML2 and YAP1-NUTM1 fusions in poroma and porocarcinoma. J Clin Investig. 2019;130:3827–32.
Davies KD, Le AT, Sheren J, Nijmeh H, Gowan K, Jones KL, et al. Comparison of molecular testing modalities for detection of ROS1 rearrangements in a cohort of positive patient samples. J Thorac Oncol. 2018;13:1474–82.
Benayed R, Offin M, Mullaney K, Sukhadia P, Rios K, Desmeules P, et al. High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no mitogenic driver alteration detected by DNA sequencing and low tumor mutation burden. Clin Cancer Res. 2019;25:4712–22.
Radovich M, Pickering CR, Felau I, Ha G, Zhang H, Jo H, et al. The integrated genomic landscape of thymic epithelial tumors. Cancer Cell. 2018;33:244–58 e10.
Petrini I, Meltzer PS, Kim IK, Lucchi M, Park KS, Fontanini G, et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat Genet. 2014;46:844–9.
Feng Y, Lei Y, Wu X, Huang Y, Rao H, Zhang Y, et al. GTF2I mutation frequently occurs in more indolent thymic epithelial tumors and predicts better prognosis. Lung Cancer. 2017;110:48–52.
Acknowledgements
The authors would like to acknowledge the Brigham and Women's Hospital Department of Pathology and the Dana Farber Cancer Institute for providing funding to support sequencing studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MV performs consulting work for Merck Pharmaceuticals. These disclosures do not apply to the current study, which is not associated with a specific source of funding. LMS reports consulting fees from Foghorn Therapeutics and LOXO Oncology and honorarium from AstraZeneca Pharmaceuticals. These disclosures do not apply to the current study, which is not associated with a specific source of funding. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Vivero, M., Davineni, P., Nardi, V. et al. Metaplastic thymoma: a distinctive thymic neoplasm characterized by YAP1-MAML2 gene fusions. Mod Pathol 33, 560–565 (2020). https://doi.org/10.1038/s41379-019-0382-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0382-x
This article is cited by
-
Atypical thymomas with squamoid and spindle cell features: clinicopathologic, immunohistochemical and molecular genetic study of 120 cases with long-term follow-up
Modern Pathology (2022)
-
Recurrent YAP1::MAML2 fusions in “nodular necrotizing” variants of myxoinflammatory fibroblastic sarcoma: a comprehensive study of 7 cases
Modern Pathology (2022)
-
A novel YAP1-MAML2 fusion in an adult supra-tentorial ependymoma, YAP1-fused
Brain Tumor Pathology (2022)
-
Loss of expression of YAP1 C-terminus as an ancillary marker for epithelioid hemangioendothelioma variant with YAP1-TFE3 fusion and other YAP1-related vascular neoplasms
Modern Pathology (2021)
-
Molecular pathology of thymomas: implications for diagnosis and therapy
Virchows Archiv (2021)