Abstract
Ductal carcinoma in situ (DCIS) is a neoplastic proliferation of mammary ductal epithelial cells confined to the ductal-lobular system, and a non-obligate precursor of invasive disease. While there has been a significant increase in the diagnosis of DCIS in recent years due to uptake of mammography screening, there has been little change in the rate of invasive recurrence, indicating that a large proportion of patients diagnosed with DCIS will never develop invasive disease. The main issue for clinicians is how to reliably predict the prognosis of DCIS in order to individualize patient treatment, especially as treatment ranges from surveillance only, breast-conserving surgery only, to breast-conserving surgery plus radiotherapy and/or hormonal therapy, and mastectomy with or without radiotherapy. We conducted a semi-structured literature review to address the above issues relating to “pure” DCIS. Here we discuss the pathology of DCIS, risk factors for recurrence, biomarkers and molecular signatures, and disease management. Potential mechanisms of progression from DCIS to invasive cancer and problems faced by clinicians and pathologists in diagnosing and treating this disease are also discussed. Despite the tremendous research efforts to identify accurate risk stratification predictors of invasive recurrence and response to radiotherapy and endocrine therapy, to date there is no simple, well-validated marker or group of variables for risk estimation, particularly in the setting of adjuvant treatment after breast-conserving surgery. Thus, the standard of care to date remains breast-conserving surgery plus radiotherapy, with or without hormonal therapy. Emerging tools, such as pathologic or biologic markers, may soon change such practice. Our review also includes recent advances towards innovative treatment strategies, including targeted therapies, immune modulators, and vaccines.
Similar content being viewed by others
The implementation of widespread mammography screening has led to significant increase in the diagnosis of ductal carcinoma in situ (DCIS), which nowadays accounts for ~20–25% of resected breast specimens [1,2,3]. DCIS is defined by the World Health Organization as a neoplastic proliferation of mammary ductal epithelial cells confined to the ductal-lobular system [4]. This proliferation is separated and guarded from the surrounding fibrovascular stroma by the basement membrane and a barricade of myoepithelial cells. This has led to the question of whether DCIS can be classified as a cancer [5]. Biologically, if DCIS remains contained in the ductal system in its pure form it cannot metastasize and hence it would not impact on the survival of women diagnosed with this lesion. However, this neoplastic process has an inherent, although not an obligate and, in reality, very low, progression to invasive carcinoma. Perhaps for this reason, the term DCIS has persisted among the healthcare and research community despite proposals for alternative terminology.
Similar to most other cancer types, the biologic behavior of DCIS is heterogenous with rapidly and slowly progressing cancer types [6].
The primary aim of DCIS treatment is to prevent local recurrence in the form of in situ or invasive disease. Recurrence has an overall mortality rate of 3.3% for DCIS (after 20 years’ follow-up, which likely represents an undetected invasive component) and up to 13.5% after an invasive recurrence [7]. A long-term outcome study of DCIS with systemic review, meta-analysis, and meta-regression analysis showed a 15-year total local recurrence rate of only 40% after a diagnosis of DCIS on excisional biopsy [8]. Among patients with recurrence, 28.1% had invasive disease, with a cancer-specific mortality rate of 18% [8]. This evidence indicates that a large proportion of patients diagnosed with DCIS will never develop invasive disease.
The main dilemma, presently, for clinicians is how to reliably predict the prognosis of DCIS and to tailor individual patient treatment accordingly [3], knowing that the natural history of DCIS shows us that even low-risk subtypes can recur after 30 years, of follow-up [9]. The spectrum of treatment spans from surveillance only, breast-conserving surgery only, to breast-conserving surgery plus radiotherapy and/or hormonal therapy, and mastectomy with or without radiotherapy.
This review will discuss the pathology of “pure” DCIS (DCIS without invasive components), including risk factors for recurrence, optimum margins, biomarkers, and molecular signatures; management of DCIS, including surgical approaches and the role of sentinel lymph node sampling, hormonal therapy, radiotherapy, and surveillance; potential mechanisms of progression of DCIS to invasive cancer; and, finally, future directions in this field.
We initially conducted a semi-structured literature review in order to discuss the above topics relating to pure DCIS. In the first instance, published studies were identified and abstracted from PubMed, conference proceedings (United States and Canadian Academy of Pathology [USCAP], American Society of Clinical Oncology [ASCO], American Society for Radiation Oncology [ASTRO], European Society for Medical Oncology, European Breast Cancer Conference, San Antonio Breast Cancer Symposium, and St. Gallen International Breast Cancer Conference), and manual searches of reference lists from systematic reviews and consensus conferences. We included articles published between January 1, 2007 and November 30, 2017, and conference abstracts published between January 1, 2015 and November 30, 2017. Selected older references were added for historical context, while newer references were also retrieved and included, if found important or complementary to the review. We reviewed abstracts to confirm relevance to the search criteria, and excluded studies of invasive breast cancer only, lobular carcinoma, non-breast ductal cancers (e.g., pancreatic ductal cancer), letters, comments, and case reports.
Epidemiology and risk factors for developing DCIS
Patients with DCIS have similar epidemiologic factors (including older age, family history of breast cancer, high mammographic breast density, and postmenopausal hormonal therapy use), genetic risk factors (BRCA1/2), and subtypes (luminal, basal, human epidermal growth factor receptor 2 [HER2]-enriched) to patients with invasive breast cancer, in keeping with the suggested role of DCIS as a precursor lesion, although not an obligate one [10,11,12,13]. BRCA1 carriers have no higher risk of DCIS, although the disease generally occurs in patients at a younger age [14].
Use of imaging in DCIS diagnosis
Recent reviews of the literature indicate that in countries where breast cancer screening programs are set up, the most common identification of DCIS is through the detection of micro-calcifications on screening mammography (70–80%) [3, 5]. Remaining cases present either as a palpable mass or as nipple discharge. The extent of the DCIS could be underestimated on mammography as not all DCIS components in the same patient are associated with calcifications. Magnetic resonance imaging has a limited role in DCIS diagnosis as it can miss low-grade lesions. Nevertheless, it is more sensitive in identifying high-grade lesions due to their high vascularity compared with low-grade DCIS.
Tomosynthesis, a new three-dimensional (3D) mammographic imaging modality, is emerging as a new screening tool that may improve breast cancer detection, mostly in invasive cancer. A recent population-based study found that 1 out of 16 cases of DCIS was detected only by tomosynthesis, compared with 28 out of 74 invasive breast cancers [15]. Ultrasound may also be used for breast cancer screening and may have a role in women with dense breast tissue [16].
Management of DCIS
Access to long-term survival data, large observational studies, and clinical trials has significantly changed the treatment paradigm of DCIS from total mastectomy to conservative excision (with or without radiotherapy and/or hormonal therapy) [7, 17]. Surveillance, that is, observation only, is being studied in clinical trials for patients with low-risk disease. However, current recommendations include the following options: mastectomy (level 2A evidence), breast-conserving surgery plus radiotherapy (level 2A evidence), and breast-conserving surgery without radiotherapy (level 2B evidence) [18, 19]. The recent NCCN Clinical Practice Guidelines in Oncology [19] state that the option of breast-conserving surgery alone may be considered where the patient and the physician view the individual as having a low risk of disease recurrence, but the guidelines remain vague about acceptable criteria for low-risk DCIS.
Surgery
The surgical approach to DCIS includes breast-conserving surgery and total mastectomy. Breast-conserving surgery alone has been suggested as an effective treatment for DCIS, with 69 and 84% of patients free of any local or invasive recurrence, respectively, at 15 years’ follow-up. Nonetheless, the addition of radiotherapy significantly improved these rates to 82 and 90%, respectively [20]. Moreover, adjuvant radiotherapy following breast-conserving surgery reduced the risk of ipsilateral invasive recurrence by ~50% at 10 and 15 years’ follow-up, compared with surgery alone [17, 20,21,22], but had no significant effect on contralateral recurrence [17]. At 20 years’ follow-up, radiotherapy decreases the risk of an ipsilateral event by 12% (10% for DCIS and 2% for invasive disease) [23]. The Eastern Cooperative Oncology Group (ECOG)–American College of Radiology Imaging Network (ACRIN) E5194 study looked at patients with either low- or intermediate-grade DCIS or high-grade DCIS <10 mm both with ≥3 mm negative margins without radiotherapy. Just over half (52%) of ipsilateral recurrences were invasive, and all recurrence events increased over time in both cohorts, without plateau. The 12-year rate of developing any ipsilateral event was 14% for low- or intermediate-grade DCIS and 25% for high-grade DCIS. These data suggest that excision alone is not sufficient [24].
Despite the above evidence, studies have shown a non-significant increase in contralateral events in patients receiving breast-conserving surgery and radiotherapy, which is also thought to be less effective in younger patients [23]. Therefore, there may be patient groups for whom radiotherapy is less effective and can be avoided, or where mastectomy is indicated. While patients with invasive local recurrence have a worse survival rate overall, local recurrence with DCIS does not appear to affect either breast cancer-specific or overall survival [20]. The addition of radiotherapy does not reduce the rate of salvage mastectomy after local recurrence compared with breast-conserving surgery alone at 10 years [25].
In patients receiving breast-conserving surgery alone, HER2 positivity has been linked with reduced time to tumor recurrence [22]. However, in patients receiving breast-conserving surgery with negative margins >2 mm, radiotherapy, and tumor bed boost, one institution observed no cases of local recurrence after 58 months, follow-up, and only 3% with contralateral recurrence (invasive and in situ) [26].
Mastectomy is offered to patients with large tumors or multifocal disease, in whom reasonable cosmesis cannot be achieved, and in younger patients with a strong family history or BRCA mutation. These patients are currently offered immediate or subsequent breast reconstruction [27]. Advocates of mastectomy have claimed that even in patients with small, low-grade DCIS, wide excision alone with margins ≥10 mm may not be adequate, since the 5-year ipsilateral local recurrence rate has been found to be as high as 12% [28].
Radiotherapy
Radiation therapy is regarded as a beneficial (and necessary by many) addition to breast-conserving surgery in the management of DCIS. A meta-analysis of four randomized clinical trials by the Early Breast Cancer Trialists’ Collaborative Group and Cochrane Collaboration showed that radiotherapy following breast-conserving surgery with clear margins was beneficial in all subgroups [29]. Radiotherapy approximately halved the risk of local recurrence, with 50% of these recurrences being invasive [17, 23, 29,30,31,32,33]. However, an analysis of 3729 patients with DCIS from four randomized trials found no significant effect of adjuvant radiotherapy on breast cancer mortality, mortality from causes other than breast cancer, or all-cause mortality after 10 years, of follow-up [29].
Radiotherapy may not be necessary in patients who have undergone breast-conserving surgery for low- or intermediate-risk lesions, according to the Van Nuys Prognostic Index (VNPI), particularly those with estrogen receptor (ER)-positive DCIS [34]. Curigliano et al. [35] found that there was a significant difference in overall and DCIS recurrence in patients with HER2-positive DCIS who underwent lumpectomy without radiotherapy vs. with radiotherapy. However, this did not have an effect on breast cancer-specific mortality. Clinical trials to date have not been able to identify a subgroup of women who did not benefit from adjuvant radiotherapy even though the degree of benefit varies across subgroups.
Population-based studies found that subsets at high risk of recurrence, for example, patients <50 years with high-grade DCIS, have a 35% risk of recurrence at 10 years after breast-conserving surgery alone. This suggests that some patients may be under-treated [36].
The addition of radiotherapy to breast-conserving surgery comes with a 0.8% risk of developing a second non-epithelial breast cancer, including leukemia and angiosarcoma, as noted in some population-based studies [37, 38]. Alternative less toxic strategies, such as accelerated hypofractionated whole-breast irradiation, that limit radiation exposure to 1–3 weeks was shown by the Ontario Clinical Oncology Group trials to have similar local control results to standard whole-breast irradiation [39]. Hypofractionated radiation was also beneficial in reducing the rates of breast edema, telangiectasia, and breast shrinkage [39, 40]. Other alternatives include irradiation of the lumpectomy cavity, and accelerated partial-breast irradiation with different modalities [41].
Hormonal treatment
The St. Gallen guidelines acknowledge the potential for hormonal therapy as effective adjuvant treatment for DCIS [18, 42, 43]. Tamoxifen has been shown to reduce both ipsilateral and contralateral events by ~30% at 10 and 15 years, follow-up [30, 44], and to reduce the risk of any breast event in ER-positive DCIS [44, 45]. Tamoxifen may also be more efficacious in patients receiving concurrent adjuvant radiotherapy [30]. Low-dose tamoxifen has also been shown to be effective, and significantly reduces any breast event and ipsilateral DCIS recurrence by ~14–34%, but not contralateral or ipsilateral invasive recurrence [44]. These effects appear to be age-related, with greater efficacy in women ≥50 years compared with those <50 years [44].
Aromatase inhibitors have also been found to be an effective treatment in DCIS. The International Breast Cancer Intervention Studies-II trial found anastrozole to be similar to tamoxifen in terms of rates of overall recurrence in patients with hormone receptor-positive disease. And while the two drugs have different safety profiles, the rates of adverse events reported were the same [42, 46]. In contrast, Margolese et al. [43] found anastrozole to have an age-dependent superiority to tamoxifen. The 10-year breast cancer-free interval was significantly higher in patients treated with anastrozole, but only in the <60 years age group. This is again in contrast to tamoxifen, which was more effective in older patients [44]. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-35 study found similar efficacy for anastrozole and tamoxifen in patients aged ≥60 years, but certain adverse events associated with tamoxifen were significantly higher in younger patients (<60 years) [46].
Surveillance only
Higher sensitivity of screening modalities and widespread implementation of breast screening programs has resulted in a significant increase in the number of women diagnosed with DCIS; based on the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (SEER) data, 15–20% of all screening-detected breast cancer are DCIS [47]. Many patients will never progress to invasive disease as <1% of cases per year progress from DCIS to invasive breast cancer [48]. Furthermore, in spite of widespread screening implementation and increased diagnosis of DCIS, the incidence of advanced breast cancer diagnosis has not decreased [49]. There is also no evidence that DCIS treatment has an effect on breast cancer-specific mortality [7]. Thus, scientists are actively investigating risk stratification tools, be it pathologic or biologic markers, to identify women with low-risk disease to spare them aggressive treatment [50] and recently Groen et al. [48] suggested that a group of low-risk patients could potentially be managed by surveillance only.
In a risk projection model, Ryser et al. [51] determined that active surveillance was a viable option, particularly for older patients and those with other substantial mortality risks. Several prospective studies are currently investigating active surveillance for low-risk lesions. The principle of comparing outcome of surveillance with standard of care is undertaken by four groups: LORD (mainland Europe), LORIS (UK), COMET (USA), and LARRIKIN (Australia and New Zealand) [48].
Surveillance alone may be a viable option in well-designed care settings with close patient follow-up. Patients under surveillance should receive careful and continuous monitoring for risk factors and signs of associated invasive breast cancer [52]. Since such a monitoring approach is still imperfect, current professional treatment guidelines still recommend surgery and radiotherapy [18, 53].
Morphologic features of DCIS and outcomes
Best practice for the processing and reporting of breast specimens with DCIS is defined in the College of American Pathologists’ protocol [54]. Items that require mandatory reporting include size/extent, morphologic features of DCIS, and margin status.
The morphologic features of DCIS that have been most consistently found to predict the risk of local recurrence in retrospective and prospective studies include high nuclear grade, presence of comedonecrosis, larger lesion size, and multifocal DCIS. Recently, DCIS-associated inflammatory infiltrate and microvessel density have also been identified as potential prognostic markers (Fig. 1). Here, we summarize results from selected studies on morphologic prognosticators, examine issues surrounding the reproducibility of DCIS pathologic feature assessment, and discuss various predictive systems for DCIS recurrence.
The significance of traditional morphologic features of DCIS as prognosticators has been examined thoroughly in three meta-analyses. In a meta-analysis by Shamliyan et al. [55], the authors examined the clinical and tumor characteristics of DCIS associated with ipsilateral DCIS or invasive recurrence, using data from five randomized controlled trials and 64 observational studies published between 1970 and 2009. In terms of tumor characteristics, tumor size was positively correlated with a higher rate of ipsilateral breast tumor recurrence, although many of the estimates were not statistically significant. Most studies defined “small” DCIS as those ≤20 mm in size. High-grade DCIS consistently showed an increased risk of ipsilateral recurrence when compared with low-grade DCIS, with a cumulative risk estimate of 2.04. Fewer studies compared the risk of intermediate-grade DCIS with that of low-grade DCIS, and the results were much less consistent. Comedonecrosis was consistently and strongly associated with ipsilateral recurrence, with the hazard ratio (HR) generally above 2.0. Interestingly, the risk of comedonecrosis appeared to be associated with treatment modality; DCIS with comedonecrosis managed by breast-conserving surgery alone was 1.16 times more likely to develop ipsilateral recurrence, compared to DCIS managed with mastectomy, skin-sparing mastectomy, or breast-conserving surgery plus radiotherapy. In a few studies with small samples, the histologic growth pattern showed an association with adverse outcome: DCIS with micropapillary, papillary, or solid architecture was more likely to develop recurrence.
The same group subsequently published another meta-analysis focusing on tumor characteristics as predictors of local recurrence [56]. The raw data were extracted from five randomized controlled trials and 36 observational studies. The results from this meta-analysis were generally consistent with the results of their previous study [55]. In addition, multifocal DCIS was also consistently found to be associated with a higher risk of ipsilateral recurrence, with a summary risk estimate of 1.95.
A meta-analysis by Zhang et al. [57] focused solely on predictors of local invasive recurrence, using data from five randomized controlled trials and 13 observational studies published between 1998 and 2014. High- and intermediate-grade DCIS, comedonecrosis, and tumor size were all inconsistently and non-significantly associated with risk of local invasive recurrence. Conversely, multifocal DCIS was associated with a significantly increased risk of local invasive recurrence with a pooled HR of 1.34. This meta-analysis did not assess the impact of treatment modality on the predictors of local invasive recurrence.
Recently, other morphologic features of DCIS have also been examined as potential prognosticators. A study by Pruneri et al. [58] examined the prevalence and clinical relevance of tumor-infiltrating lymphocytes (TILs) in the periductal stroma in 1488 patients with DCIS. There were 35.1% of DCIS cases with <1%, 58.3% with 1–49%, and 6.5% with >50% of TILs in the periductal stroma. A high level of stromal TILs was significantly associated with other high-risk features in DCIS, such as HER2-positive or triple-negative intrinsic subtypes, high nuclear grade, and necrosis. However, no significant association between the level of TILs and the incidence of ipsilateral breast cancer events (in situ or invasive) was identified after a median follow-up of 8.2 years.
In another study by Campbell et al. [59], the immunohistochemical characteristics of the DCIS-associated immune infiltrates and clinical outcomes of 52 cases of high-grade DCIS were compared with those of 65 cases of non-high-grade DCIS. TILs identified within both the DCIS lesion and stromal TILs were assessed. The investigators found that high-grade DCIS had significantly higher percentages of FoxP3-positive or human leukocyte antigen–antigen D-related (HLA-DR)-positive cells, CD68-positive and CD68/proliferating cell nuclear antigen-positive macrophages, CD4-positive T cells, CD20-positive B cells, and total TILs compared with non-high-grade DCIS. In addition, cases with low numbers of activated CD8/HLA-DR-positive T cells, high numbers of non-activated CD8/HLA-DR-positive or CD8-positive/HLA-DR-negative T cells, as well as high numbers of CD115-positive macrophages in TILs, were associated with high risk of local and metastatic recurrences, and a prognostic model using these three immune cell populations had an accuracy of 87% (sensitivity, 76%; specificity, 89%).
Finally, in a recent study by Knopfelmacher et al. [60], the presence of dense chronic inflammatory infiltrates surrounding DCIS was found to be significantly associated with a high Oncotype DX® Breast DCIS Score™ (Genomic Health Inc., Redwood City, CA, USA). In this study, a dense inflammatory infiltrate was defined as circumferential or near circumferential (>75% of circumference) cuffing of DCIS ducts by lymphocytes or plasma cells at least three cell layers in thickness. However, the results were based on only 46 cases of DCIS, and no correlation with clinical outcome was provided. Inflammation has also been studied in the context of the so-called “regressive” change in high-grade DCIS, which has been defined by some authors as progressive ductal fibrosis and inflammation ultimately leading to obliteration of the duct. A few studies have reported an association between these changes and invasive recurrence and larger volumes of DCIS.
The association of microvessel density with DCIS outcome is controversial. Microvessel density is commonly assessed by counting the number of lymphovascular channels identified by morphology and/or immunohistochemistry in the stroma immediately surrounding DCIS. While some studies have identified an association between high microvessel density and other high-risk DCIS features, such as intermediate–high nuclear grade, comedonecrosis, mitotic activity, and HER2 and p53 overexpression [61,62,63,64], as well as an increased risk of local recurrence [65], a more recent study by Adler et al. [66] found no association between microvessel density and DCIS tumor characteristics or clinical outcomes.
A significant barrier to using the nuclear grade of DCIS as a prognosticator is the low level of diagnostic agreement observed in low-grade DCIS. Nuclear grading of DCIS is based on a modified Lagios nuclear grading system [54]. A recent study by Onega et al. [67], involving 115 pathologists interpreting a test set of 240 cases of low- and high-grade DCIS, found that agreement with reference diagnoses was 83% for high-grade DCIS but only 46% for low-grade DCIS.
Predictive systems that combine various tumor and clinical characteristics to better prognosticate DCIS have been developed. The Van Nuys Classification was first developed in 1995 using a combination of nuclear grade and necrosis to predict DCIS local recurrence risk [68]. Tumor size and margin width were subsequently added to create the VNPI [68]. Finally, age at diagnosis was added to develop the University of Southern California (USC)/VNPI [69]. The USC/VNPI assigns a score of 1–3 to each of the four prognostic factors: 1 = lesions with the best prognosis; 3 = lesions with the worst prognosis [70]. A combined score ranging from 4 (least likely to recur) to 12 (most likely to recur) is then calculated [70]. A retrospective study by the Van Nuys investigators examined the validity of the USC/VNPI in 949 patients with pure DCIS treated with breast-conserving surgery (345 with excision and radiation therapy, and 604 with excision alone) and proposed treatment recommendations for each score in order to achieve a local recurrence rate of <20% at 12 years [70]. The authors found that patients with a combined score of 4, 5, or 6 could be safely treated with excision alone, with a local recurrence rate of ≤6% at 12 years. For patients who scored 7, but had a margin width of ≥3 mm, the local recurrence rate was <20% at 12 years with excision alone. Conversely, patients who scored 7 but with <3 mm margins, scored 8 with ≥3 mm margins, or scored 9 with ≥5 mm margins should be treated with postoperative radiation to achieve the <20% local recurrence requirement at 12 years. Finally, mastectomy was required for patients who scored 8 with <3 mm margins, scored 9 with <5 mm margins, or scored 10, 11, or 12 in order to keep the local recurrence rate <20% at 12 years [70].
The Van Nuys investigators also examined the validity of the USC/VNPI in 496 patients with pure DCIS treated with mastectomy alone. They found that all 11 patients who experienced recurrence had scored 10–12 using the USC/VNPI. In addition to the four prognostic factors already included in the USC/VNPI, multifocal disease and comedonecrosis were also identified as additional individual risk factors that predicted recurrence post mastectomy [71]. Despite the above, results from external validation studies are inconsistent, and several studies failed to show additional discriminatory power using the Van Nuys Classification or its variants compared with individual risk prognosticators [72,73,74,75,76]. The meta-analysis by Shamliyan et al. [55] also examined the Van Nuys Classification and its variants. In general, women with high scores had worse outcomes, although the association between score and risk of ipsilateral breast tumor recurrence was not linear.
In 2010, data from 1868 patients treated with breast-conserving surgery for DCIS were retrospectively analyzed, and a nomogram for predicting the risk of local recurrence for DCIS at 5 and 10 years was created. The nomogram included age at diagnosis, family history, mode of DCIS detection, use of adjuvant radiation, use of adjuvant endocrine therapy, nuclear grade, necrosis, margin status, number of excisions, and year of surgery as variables. An internal validation study showed that this model demonstrated reasonable discrimination with a concordance index (C-index) of 0.7 (0.69 with bootstrap validation), similar to C-indices for other cancer prediction models [77]. Subsequent external validation studies on this DCIS nomogram demonstrated similar results, with a C-index ranging from 0.63 to 0.68 [78,79,80].
Recently, a clinical risk score was published by the National Comprehensive Cancer Network (NCCN). Data from 2762 patients treated with breast-conserving surgery for DCIS were examined: 356 with excision alone, 1251 with excision and radiation, 154 with excision and hormonal therapy, and 1001 with excision, radiation, and hormonal therapy. Age ≤50 years at DCIS diagnosis, presence of comedonecrosis, and ER-negative status were found to predict ipsilateral recurrence within 5 years. These variables were then used to create the risk score and combined to form risk groups. The 5-year likelihood of ipsilateral recurrence with no adjuvant therapy was 9% for the low-risk group, 23% for the intermediate-risk group, and 51% for the high-risk group. The C-index of the risk score was 0.74 (0.76 with cross-validation). This risk score was externally validated on a separate DCIS population of 301 patients, with a C-index of 0.67. This risk score has several potential benefits over the previously discussed nomogram: by excluding treatment and margin status as variables, this risk score can be used to predict the risk of ipsilateral recurrence in patients with DCIS, who have achieved negative surgical margins after breast-conserving surgery, in order to guide subsequent adjuvant treatment decisions [77]. The risk score is also easy to use [81]. More external validation studies are needed to confirm the validity of this risk score.
Margin assessment in DCIS
Clinical impact of margin status
The current consensus recommendations on margin assessment for patients with DCIS undergoing breast-conserving surgery were published jointly by the Society of Surgical Oncology, ASTRO, and ASCO [53], and were recently endorsed by a panel of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer [18]. The current evidence and recommendations can be summarized as follows:
Positive margins
The cumulative evidence indicates that a positive margin (defined as DCIS on the inked surface) is an independent risk factor for tumor recurrence in a multivariate analysis [20, 82]. Radiotherapy decreases, but does not nullify, the risk of recurrence of DCIS in patients with positive margins [53, 83]. For these reasons, re-excision is indicated in this scenario.
Negative margins ≥2 mm
Wide negative margins (≥2 mm) significantly reduce the risk of ipsilateral recurrence in patients receiving whole-breast radiotherapy [53]. A meta-analysis by Marinovich et al. [84] found a statistically significant decrease in recurrence for 2 mm margins compared with 0–1 mm margins. The same study showed no further recurrence risk reduction for widths >2 mm (e.g., 5 or 10 mm). Thus, in the context of adjuvant radiotherapy, a width of ≥2 mm is the current recommendation for margin clearance; this parameter is increasingly being accepted as a determinant against re-excision [85].
In patients foregoing radiotherapy, the current consensus also recommends a negative margin of ≥2 mm. A large study by Van Zee et al. [82] found recurrence rates of 16% for margins >10 mm, compared with 23% for margins 2.1–10 mm, and 27% for margins 0–2 mm. These data suggest that wider margins may benefit patients not undergoing radiation. Nonetheless, the consensus guidelines do not include a definitive recommendation on a margin threshold ≥2 mm in this patient subset.
Negative margins 0–1.9 mm
In patients undergoing whole-breast radiotherapy, a close negative margin (0–1.9 mm) is not by itself an indication for re-excision, and other prognostic factors such as patient age, residual mammographic abnormalities, DCIS extent, and grade should be considered [53, 82, 86]. Indeed, there is recent evidence showing that in the context of adjuvant radiotherapy, the risk of recurrence in patients with 0–1 mm margins and those with ≥2 mm margins is similar [82, 87]. Conversely, patients with close negative margins who do not receive radiotherapy have significantly higher rates of regional recurrence compared with those with ≥2 mm negative margins [87]. Therefore, in this patient population, re-excision is justified.
It is important to note that a negative margin does not guarantee the absence of residual DCIS. The presence of uninvolved tissue between foci of DCIS is a well-documented phenomenon; [88] furthermore, it has been shown that the prevalence of residual carcinoma on re-excision is inversely proportional to the margin width [89, 90]. The evidence suggests that radiotherapy plays a role, halting the progression of any residual DCIS towards recurrence.
In mastectomy specimens performed for DCIS, the rate of positive margins varies among studies. In a study by Fitzsullivan et al. [91], 0.6% of mastectomies had positive margins and 11.7% had margins <3 mm. In this study, a close margin was an independent risk factor for loco-regional recurrence. A separate population-based analysis by Klein et al. [92] showed a much higher rate of margins ≤2 mm (43.8%). Furthermore, the study found no association between ≤2 mm margins and chest wall recurrence.
Pathologic evaluation of margins in excisions for DCIS
Specimen handling
Proper handling and processing of breast-conserving surgery specimens is an essential requirement for optimal margin evaluation. Use of X-rays is a significant part of pathologic examination of DCIS specimens to ensure complete examination of disease extent, multifocality, and margins. Multicolor margin labeling has become routine practice in most laboratories. Ideally, orientation and inking by the pathology team should occur immediately after excision, as post-excision handling can distort the landmarks for margin identification. It has been shown that compression at the time of radiography distorts the specimen and may artificially decrease the distance of the lesion to the deep margin [93]. Orientation by the pathologist based solely on the standard two-suture approach can result in incorrect margin identification in a significant proportion of cases [94]. Moreover, margin identification and inking by the pathologist appears to be inferior to identification and inking by the surgeon in terms of margin-positivity rates and residual disease on re-excision [95].
All the margins must be represented in the tissue sections submitted to histology. While en face (shaved) margins have been advocated because they allow examination of a larger margin surface area [90, 96], they preclude distinction between close (0–1 mm) and wide (≥2 mm) negative margins, which is required to determine the need for re-excision as outlined above. Therefore, perpendicular margins are largely preferred. Of note, there is evidence that obtaining additional shaved margins as well as the main resection decreases the rate of positive margins [97, 98]. If this approach is practiced by the surgeon, the additional shaved margins should be sectioned perpendicularly with the final margin properly identified (inked), and submitted generously or entirely akin to any other re-excised margin.
Margin interpretation
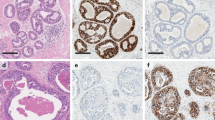
The distance between DCIS and each margin needs to be measured in millimeters. In our practice, we measure the distance through the microscope (not with the naked eye) using a ruler, allowing fractions of a millimeter to be reported (e.g., 1.2 mm). Ideally, the tissue margin has an even and smooth edge uniformly displaying ink and cautery effect. It is important to avoid gaps or indentations in the tissue, since these almost never represent true margins; even if they display ink and/or cautery effect, they should be interpreted with caution. Correlation with the specimen photograph and the macroscopic description can help in this scenario (Fig. 2).
Margin assessment in DCIS. a Sections should always be correlated with the gross diagram to properly identify margins; the diagram must allow identification of all the margins present in each section, and any tissue edge not representing a margin must be labeled (see upper-left aspect). b Positive margin, with DCIS at the inked margin surface (see inset). c True margins must have ink and (if electrocautery is used by the surgeon) cautery effect, and should follow the outer contour of the section smoothly. Gaps in the tissue, due to transection by the surgeon or post-resection handling, can be recognized if the section is properly oriented. d Wide negative margin (>2 mm). e Artificial gaps should be avoided for margin distance measurement purposes; if the closest margin is partially detached from the main tissue section, it is important to subtract the artificial gap as measuring the entire distance would result in overestimation. f DCIS touching tissue edges that do not represent a true margin but an artificial gap created at the time of excision (the so-called “hesitation mark”, corresponding to wide gap in c). DCIS, ductal carcinoma in situ
Margin reporting
The current College of American Pathologists’ protocol for DCIS [54] outlines the reporting of margins as:
(a) Positive for DCIS: List all the positive margins. The extent of margin involvement can also be reported, as it correlates with the likelihood of residual disease on re-excision [99, 100]. Two systems have been described: Sigal-Zafrani et al. [99] categorized positive margins as focal (1 mm of involved margin surface length), minimal (1–15 mm), or extensive ( ≥15 mm). Neuschatz et al. [100] categorized positive margins as focal (margin involvement in 1 section, single focus), minimal (2–4 sections or 1 low-power field), moderate (5–7 sections or 2–4 low-power fields), or extensive ( ≥ 8 sections or > 4 low-power fields).
(b) Uninvolved by DCIS: Specify the closest margin and its margin width. Reporting the distance to additional negative margins is optional. In our practice, we report the margin width of any margin 10 mm, which may be useful in patients not receiving radiotherapy until further evidence allows for more accurate thresholds. The width can be provided as a precise measurement in millimeters or as a “greater than” or “less than” estimation. The former method is preferred; if the latter is used, it is important to report the estimation around the 2 mm threshold).
Sentinel lymph node sampling in DCIS
Based on retrospective evidence and informal consensus, ASCO recommends considering the use of sentinel lymph node biopsy in women with DCIS undergoing mastectomy [101]. The rationale is that a proportion of cases with preoperative DCIS diagnoses will have an invasive carcinoma on final excision, and that the mastectomy procedure alters the anatomy precluding subsequent sentinel lymph node mapping. An invasive carcinoma component explains most, but not all, cases of sentinel lymph node metastases in these patients as demonstrated in a meta-analysis by Ansari et al. [102], which showed that the positivity frequency of sentinel lymph node involvement in patients with pre-excision diagnosis of DCIS was 7.4, and in those with final (post-excision) diagnosis of DCIS was 3.7. Similarly, the prevalence in the literature of sentinel lymph node metastases in patients with pure DCIS after mastectomy ranges 0.4–13.7% [103,104,105,106,107,108]. It is presumed that these patients harbor an invasive tumor undetected by pathologic evaluation. Most nodal metastases are micrometastases and isolated tumor cells; however, macrometastases and micrometastases can also occur (0.9 and 1.5% of all patients with pure DCIS, respectively) [109]. Given these relatively low but clinically relevant rates, most authors in the field recommend the use of sentinel lymph node biopsy at the time of mastectomy in agreement with treatment guidelines.
Sentinel lymph node biopsy is generally not recommended in patients undergoing breast-conserving surgery for DCIS, as subsequent sentinel lymph node mapping is still feasible if indicated by the final pathology [110, 111].
Several additional factors may be considered in the decision to sample sentinel lymph nodes; these include the extent of DCIS, mass-forming DCIS, nuclear grade on biopsy, and the number of preceding excisions [110].
Biomarkers
Immunohistochemistry markers
There has been a pressing need for identification of prognostic and predictive biomarkers in DCIS, particularly in the detection of patients destined to progress to invasive breast cancer and those who might be spared adjuvant treatment. Unfortunately, most studies on pure DCIS are underpowered with <30–50 cases, while others are diluted by inclusion of cases with an invasive component. Moreover, variable treatment modalities and different scoring methods of immunohistochemistry markers further limit the impact of these studies.
ER expression in pure DCIS is similar to that in invasive breast cancer: 68.7% (range, 49–96.6%) based on a comprehensive literature review by Lari and Kuerer [112]; it is also highly concordant with coexisting invasive breast cancer [113]. ER-positive DCIS has a higher risk of invasive recurrence [114, 115], while ER-negative/HER2-positive DCIS has been found to be an independent factor for local in situ recurrence in a minority of studies [115, 116]. Several studies have examined ER expression in combination with other immunohistochemistry markers: an ER/HER2/Ki-67-positive profile can be predictive of DCIS recurrence (hazard ratio (HR) 5.8; 95% confidence interval [CI], 2.4–14); [117] and triple-negative DCIS is associated with a higher risk of local recurrence [13]. The results of two clinical trials, UK/ANZ and NSABP B-24, suggest ER status to be a weakly prognostic biomarker for local recurrence and a strong predictor of response to endocrine therapy to reduce local recurrence [30, 118]. ER-negative status has been found to be a statistically significant predictor for ipsilateral recurrence within 5 years (P = 0.0032) in a cohort of 2762 patients with DCIS treated with breast-conserving surgery with negative margins at NCCN centers [81]. Moreover, ER status, together with age and comedonecrosis, were used to develop a risk score that stratifies patients with DCIS who have not undergone adjuvant treatment into low-, intermediate-, and high-risk groups with 5-year likelihood of recurrence of 9% (95% CI, 5–12), 23% (95% CI, 13–32), and 51% (95% CI, 26–75), respectively; the results were cross-validated in 301 patients from the Kaiser Permanente Northern California DCIS cohort [81].
Progesterone receptor (PgR) expression in DCIS is also similar to that in invasive breast cancer, with a mean expression rate of 59.6% (range, 40–83.3%) [112]. Only single studies were able to show significant correlation between PR expression and risk of recurrence [116, 119].
Androgen receptor (AR) expression in DCIS is common (mean 65.8%; range, 37–81%) [112]. AR expression was found to be significantly higher in DCIS associated with invasive breast cancer than in pure DCIS (94 vs. 61–80%, respectively) [120, 121], and much higher (93 vs. 23–30%) compared to ER and PgR in high-grade DCIS [122]. Moreover, when comparing high-grade DCIS and high-grade invasive ductal carcinoma, the patterns of AR and ER or PgR co-expression were different between in situ and invasive carcinoma (AR-positive/ER-negative: 60% DCIS vs. 39% invasive ductal carcinoma; AR-negative/ER-negative: 7% DCIS vs. 43% invasive ductal carcinoma) [122]. These marked differences were not observed in non-high-grade subgroups, suggesting that AR–ER/PgR correlations may play an important role in the invasive transformation of high-grade DCIS. In a population of patients with DCIS treated with surgery and adjuvant radiotherapy, AR status was found to be an independent prognostic factor in multivariate analyses (HR 1.1; 95% CI, 1.01–1.11) [123]. However, among 43 patients with pure DCIS treated with surgery alone, AR expression was not found to be prognostic, while an AR:ER ratio at 1.13 showed a sensitivity of 75% and a specificity of 94% in predicting any recurrence [124].
High proliferative index as assessed with Ki-67 has been shown to be predictive of local non-invasive [21, 125], and any local [126, 127], recurrence. A recent meta-analysis also found Ki-67 to be predictive of both invasive and non-invasive recurrence [126]. Phosphohistone-H3 (pHH3) has been suggested to be superior to Ki-67 in assessing mitotic activity for its high interobserver concordance and reduced time needed for interpretation [128].
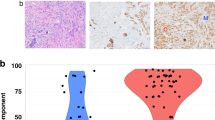
HER2 overexpression in DCIS is more frequent than in invasive breast cancer (mean, 40.1%; range, 9–67%) (Fig. 3) [112], but is highly concordant with coexisting invasive breast cancer [113]. HER2-positive DCIS is associated with higher rates of local recurrence [129]. In a study of 141 patients with DCIS treated with excision alone, HER2-positivity was found to be significantly associated with reduced time to recurrence (P = 0.028) [22]. Moreover, HER2 overexpression, either alone or in combination with high Ki-67, has been shown to result in an increased risk of non-invasive local recurrence and lower risk of invasive recurrence [21, 114, 117, 130, 131]. Interestingly, in a cohort of HER2-positive DCIS, retinoblastoma loss was associated with a further increased risk of recurrence and also significantly associated with progression to invasive breast cancer [130].
a DCIS (right) associated with invasive ductal carcinoma (left). b Overexpression of HER2 oncoprotein in DCIS (score 3+ in 100% of DCIS), contrasting with the absence of overexpression (score = 0) in the invasive component (HER2 by immunohistochemistry, clone 4B5). DCIS, ductal carcinoma in situ; HER2, human epidermal growth factor receptor 2
When using ER, PgR, or HER2 by immunohistochemistry as surrogates to classify DCIS into molecular subtypes, Williams et al. [132] found them to be predictive of both any and invasive recurrence in a cohort of 314 women with primary DCIS. For overall recurrence, luminal B (HR 5.1; P = 0.001), HER2-positive (HR 6.5; P < 0.001), and triple-negative (HR 3.3; P = 0.028) DCIS had higher risk compared to luminal A DCIS. The same applied to invasive recurrence: luminal B (HR 13.4; P = 0.014), HER2-positive (HR 11.4; P = 0.027), and triple-negative (HR 10.3; P = 0.031) DCIS [132]. Similarly, Han et al. [129] observed higher rates of local recurrence among luminal B (40%) and HER2-positive (38%) DCIS compared with luminal A (21%) and triple-negative (15%) DCIS in a cohort of 180 patients.
In one of the largest analyses of DCIS studies to date (N = 1162), immunohistochemistry performed on blocks from 329 patients in a population-based cohort found that p16/COX-2/Ki-67-positive DCIS profile was a strong predictor of subsequent invasive recurrence (HR 2.2; 95% CI, 1.1–4.5); [117] subsequent DCIS was highest for women with either ER-negative/HER2-positive/Ki-67-positive (HR 5.8; 95% CI, 2.4–14) or p16-positive/COX-2-negative/Ki-67-positive DCIS (HR 3.7; 95% CI, 1.7–7.9). Moreover, the same authors found DCIS with a p16-positive/COX-2-positive/ER-negative/HER2-positive profile to be at highest risk of metastatic regional or distant recurrence at 10 years (HR 22.5; 95% CI, 13.8–48.1) [115].
A recent publication by a Swedish group has introduced a promising immunohistochemistry-based tool for predicting radiotherapy benefit. In the SweDCIS study of radiotherapy benefit at 10 years, based on PgR, FOXA1, HER2, Ki-67, COX-2, SIAH2, and p16INK4a expression by immunohistochemistry, together with clinical-pathologic factors, the authors developed a decision score that stratified patients into low-risk and elevated-risk groups [133]. The score was found to be both prognostic and predictive of radiotherapy benefit with 9% absolute radiotherapy benefit in the elevated-risk group and no benefit in the low-risk group [133]. The study has been cross-validated with highly consistent results [134].
p53 by immunohistochemistry highly correlated with the presence of TP53 mutations, which further showed significant positive correlation with HER2 amplification [135]. p53 together with Ki-67 have been found to be predictors of coexisting invasive breast cancer [136]. Also, p53 overexpression has been found to be predictive of any local recurrence [137, 138].
In a proof-of-concept clinical trial of 95 women with pure DCIS randomized to exemestane with or without celecoxib (a COX-2 inhibitor), COX-2 was found to be an independent predictor of early relapse, which was further confirmed on a validation set of 58 patients (HR 3.9; 95% CI, 1.8–8.3; P = 0.002) [139]. However, in another pre-surgical study of 90 women with ER-positive DCIS, 77% of whom had COX-2-positive disease, there was no change in apoptosis or proliferation in response to celecoxib [140].
Jiang et al. [141] investigated IGF-IR, Rap1, and Vav2 as potential biomarkers of DCIS. The expression of the IGF-IR and Rap1 was significantly elevated in ER-positive DCIS, but the level of expression did not differ depending on the presence of concurrent invasive breast cancer. IGF-IR expression was also increased in HER2-positive DCIS. Vav2 expression, however, while not elevated in pure DCIS, was associated with the presence of concurrent invasion, with high-Vav2 DCIS more than twice as likely to progress to invasive breast cancer than low-Vav2 DCIS (odds ratio 2.42; 95% CI, 1.26–4.65; P = 0.008) [141].
Expression of NY-ESO-1, one of the most immunogenic cancer/testis antigens, which is able to induce spontaneous humoral immunity, and found to be present mostly in triple-negative breast cancer [142,143,144], was found to be indicative of good prognosis in DCIS in a small cohort of 42 patients [145].
P21, cyclin D1, calgranulin, psoriasin, and c-myc, either alone or in combination, were not found to be significant predictors of recurrence [21, 146].
Overall, ER, PgR, HER2, and Ki-67, as well as AR for high-grade DCIS, appear to be the strongest immunohistochemistry predictors of recurrence. The findings from the population-based studies from the University of California [115] and the SweDCIS trial [133, 134] using ER, PgR, HER2, Ki-67, COX-2, FOXA1, SIAH2, and p16 signatures are very promising, but guidelines regarding unified cut-offs and scoring methods are needed.
To conclude, the only biomarker that should be used in clinical practice is ER in patients considered for adjuvant endocrine therapy, and generally only performed on excisions rather than core biopsies.
Molecular signatures in DCIS
Validated prognostic and predictive multigene molecular assays in pure DCIS are limited. More importantly, no molecular signature is currently capable of successfully identifying patients at specific risk of an invasive ipsilateral breast event (invasive recurrence), women whose risk is not further reduced by radiotherapy, or those at such minimal risk that adjuvant therapy can be safely omitted.
The only clinically validated and commercially available multigene signature is the Oncotype DX Breast DCIS Score, which costs ~4000 USD. The assay is based on the expression of 12 genes derived from the 21 genes used for calculating Oncotype DX Recurrence Score for early invasive breast cancer; seven cancer-related (Ki-67, STK15, survivin, CCNB1, MYBL2, PgR, and GSTM1) and five reference (ACTB, GAPDH, RPLPO, GUS, and TFRC) genes [147]. The DCIS Score can be used to quantify the 10-year risk of any ipsilateral breast event (local or invasive recurrence) following diagnosis of DCIS without radiotherapy.
The DCIS Score was first validated by the ECOG [147] in a prospective, non-randomized, clinical trial with two groups of DCIS patients enrolled between 1997 and 2002. The first group consisted of low- or intermediate-risk DCIS (tumor size ≥25 mm) and the second group consisted of high-risk DCIS (tumor size ≥10 mm). Treatment for all patients included breast-conserving surgery and wide clear margins (>3 mm). A subset of patients were treated with adjuvant tamoxifen. The DCIS Score (based on the 12-gene assay) was used for the three pre-specified risk groups. The 10-year rates for developing an ipsilateral breast event were 10.6, 26.7, and 25.9% for the low-, intermediate-, and high-risk groups, respectively (log-rank P = 0.006). The corresponding 10-year rates for developing an invasive ipsilateral breast event were 3.7, 12.3, and 19.2%, respectively (log-rank P = 0.003). In contrast, the Recurrence Score (based on the 21-gene assay) was not significantly associated with developing an ipsilateral breast event.
The DCIS Score was further independently validated in a retrospectively identified population-based cohort of patients with negative margins who did not receive adjuvant radiotherapy between 1994 and 2003 (Ontario Cohort) [148]. In this cohort, negative margins were defined as no ink on tumor, and tissue was available for analysis in 50% of the cohort. In the pre-specified primary analysis, DCIS Score was significantly associated with risk of ipsilateral breast event both in patients with ER-positive DCIS (HR 2.3; 95% CI, 1.41–3.59; P < 0.001) and irrespective of ER status (HR 2.2; 95% CI, 1.43–3.22; P < 0.001), although close to 95% of the tumors were ER-positive. Excluding individuals with multifocal DCIS, the 10-year rates of local recurrence for the low-, intermediate-, and high-DCIS Score groups were 9.7, 27.1, and 27.0% (log-rank P < 0.001), respectively. The corresponding 10-year rates of invasive recurrence were 5.6, 16.7, and 16.3% (P = 0.02). As with the ECOG-ACRIN E5194 study, the Ontario Cohort analysis was best fitted in a dichotomous model with low-risk DCIS Score vs. intermediate-/high-risk taken together. Although the ECOG-ACRIN E5194 study included mainly low-risk DCIS cases and the later cohort included all-comers, the low-risk DCIS Score entailed about 10% risk of an ipsilateral breast event, or 3.7–5.6% risk of an invasive event at 10 years, in both populations. Multivariable analyses combining both studies found that in addition to the DCIS Score, age at diagnosis and tumor size were also significant predictors of local recurrence. In addition, multifocality emerged as a marker of increased risk in the Ontario Cohort and postmenopausal status emerged as a marker of reduced risk in the ECOG-ACRIN E5194 study.
Comparison between histopathologic features and DCIS Score
Since the Oncotype DX Breast DCIS Score assay became commercially available there have been few publications correlating the score with traditional histopathologic prognosticators. A single institution study including 46 cases of DCIS and available DCIS Score found an association between high-ER/PgR expression, low mitotic rate, and low DCIS Score, as well as dense periductal chronic inflammation [60]. In another single institution study including 37 patients with DCIS and available DCIS Score, low DCIS Score was associated with low nuclear grade and a lower rate of adjuvant radiotherapy (P < 0.008) [149]. However, in about a fifth of cases, the DCIS Score was considered unexpected in relation to the histopathologic findings, that is, high nuclear grade with comedonecrosis and a low DCIS Score, or hormone receptor discrepancies. In this study, 5 of the 15 patients made the decision to forgo radiotherapy based on the DCIS Score results.
Integration of DCIS Score in current practice
At present, there is limited experience with the clinical utility of the DCIS Score. A previous study by Alvarado et al. [150] evaluated the influence of the test in 10 centers in the US in 115 patients. The DCIS Score led to a change in the treatment recommendation in 36 patients (31.3%); 26 patients (22%) had a change to no radiotherapy, and 10 patients (8.7%) had a change to being recommended radiotherapy. A study analyzing the survey responses of 539 surgeons and radiation oncologists in the US assessed the potential utility of the DCIS Score [151]. Clinicians were presented with the hypothetical case of a 65-year-old woman with ER-positive DCIS who had a lumpectomy with negative margins (>10 mm), and agreed to adjuvant tamoxifen therapy [151]. Among the surveyed clinicians, approximately one-third would have ordered the DCIS Score [151]. A prospective non-randomized trial is open for enrollment in Canada and is designed to assess the impact of the DCIS Score on clinical practice, in particular, the ability of the DCIS Score to change physician recommendations for radiotherapy and the effect on patient radiotherapy treatment preference in low- or intermediate-risk pure DCIS (DUCHESS) [152].
Given the high cost of multigene assays, a cost-effectiveness analysis based on a Markov model simulating 10-year outcomes for women aged 60 years eligible for the ECOG-ACRIN E5194 study was conducted [153]. The study compared five different treatment strategies with the reference strategy (surgical excision with no radiotherapy); two of the strategies incorporated DCIS Score testing either for all patients or selected patients with intermediate- and high-grade DCIS, and recommended radiotherapy for patients with intermediate or high DCIS Scores. The strategies using the DCIS Score lowered the proportion of women undergoing radiotherapy per ipsilateral breast event prevented. However, no strategy incorporating the DCIS Score was cost-effective. Based on these data, it has been suggested that a recommendation to use the DCIS Score could be made by multidisciplinary teams in the context of a tumor board or direct communication [154].
It is important to note that the DCIS Score assay is prognostic rather than predictive of response to radiotherapy; it informs about the risk of ipsilateral breast events (in situ or invasive) and may assist in the decision-making process for clinicians and patients with regards to possible further treatment options. However, current estimates of in situ and invasive local recurrence risks associated with the DCIS Score do not adjust for other independent prognostic factors such as age, tumor size, comedonecrosis, and multifocality. Nevertheless, other available algorithms such as the recently suggested Clinical Score [81], DCIS nomogram [77], and the USC/VNPI [69] are based only on population averages and do not offer a truly individualized risk estimate. Therefore, there is a need to establish a multiparameter algorithm combining clinical-pathologic and molecular characteristics.
Consideration for block selection
The DCIS Score is performed on tissue fixed in neutral-buffered formalin and embedded in paraffin. The optimal block for testing should include the highest grade of DCIS, preferably with the greatest dimension on a single slide. In our experience, ≥2 mm of contiguous linear lesional tissue, at least in one dimension, is required. Consideration should also be made to avoid foci with extensive comedonecrosis, leading to diminished cellularity. Hemorrhage and adipose tissue do not need to be minimized as they contain little RNA and thus do not significantly impact the assay. In some cases, the core biopsy may be more suitable for testing than the excisional specimen, particularly when a relatively small lesion is assessed by vacuum-assisted biopsy. Occasionally, we encounter requests for tissue submission in infrequent lesions of debatable classification, such as encysted papillary carcinoma and solid papillary carcinoma without conventional invasion. While the recommendation based on the current World Health Organization classification is to treat these lesions similarly to conventional in situ carcinoma [4], it should be noted that the prognostic role of the DCIS Score in these specific lesions has not been established.
Other molecular markers
Relatively low rates of ipsilateral breast events (particularly invasive events), variability in extent of surgical excision, use of adjuvant radiotherapy, and use of endocrine therapies pose a substantial challenge for discovery or validation studies on prognostic and predictive biomarkers. There is a paucity of pure DCIS cohorts with long-term outcomes and sufficient patient numbers or available tissue blocks for studies to be adequately powered to allow multivariate analysis. This situation has led many investigators to design studies comparing molecular features of DCIS components with those of paired invasive components, or comparison of pure DCIS cases with DCIS components in cases of stromal invasion. These scenarios may represent separate biologic entities and therefore differ from the biology of the subset of pure DCIS cases that develop in situ and invasive ipsilateral breast events, and, as such, are not optimal investigational models. Generally, profiles of DCIS components are remarkably similar to the invasive disease when both components are analyzed, including copy number variation, CCND1, MYC, and genes related to pathways of angiogenesis, cell–cell adhesion, epithelial-to-mesenchymal transition, and the extracellular matrix [155]. The expression of mRNA and microRNA is different when normal tissue is compared with DCIS [156]. These studies are hypothesis-generating and putative markers require proper validation.
Potential mechanisms of progression from DCIS to invasive breast cancer
Intensive research activities are focused on identifying potential biologic drivers of disease progression from in situ to invasive disease. Below, we discuss some potential mechanisms and theories.
In other organs such as the colon or cervix, a linear progression from low- to high-grade intraepithelial lesion and then into invasive carcinoma is well documented. In the breast it has been noticed that parallel progression from low-grade DCIS to low-grade invasive carcinoma and high-grade DCIS to high-grade invasive carcinoma is frequently observed, although not exclusively. The distinct low- vs. high-grade progression pathways have been studied by different methodologies including immunohistochemistry and molecular techniques.
Through loss of heterozygosity, comparative genomic hybridization, gene expression profiling, next-generation sequencing, and proteomic analyses, various tissue components have been implicated as having a role in the progression from DCIS to invasive carcinoma [157]. These refer to factors related to proliferation and cell-cycle regulation, regulators of cell-to-cell interaction, regulators of extracellular matrix, and regulators of angiogenesis.
A limitation of the research in this field is the frequent confusion between the study of mechanisms of progression and markers of invasive recurrence. Examining progression is very challenging because most patients with DCIS are treated by breast-conserving surgery with or without radiotherapy or systemic therapy. Thus, there are no available opportunities to study progression in vivo without therapeutic intervention. Moreover, there are limited adequate preclinical models available.
Future directions and ongoing research
Active research focuses on the mechanisms of transition from in situ to invasive ductal carcinoma, and the discovery of prognostic and predictive markers for risk stratification and treatment guidance, as well as possible targets for therapy.
Although efforts to identify patients who could be spared aggressive treatments have been extensively advanced, there is also a need to identify patients who have higher risk of progression under standard treatment. For these patients, finding new targets for systemic therapy are required. Immune mechanisms such as those related to immune surveillance or vaccination are promising routes to explore. For instance, high levels of TILs and CD8-positive lymphocytes are associated with spontaneous “healing” in high-grade, HER2-positive comedo DCIS [158]. Programmed death-ligand 1 (PD-L1) expression may be a useful immunologic stratification marker in DCIS. PD-L1 expression is not linked to DCIS cells themselves, but to TILs within DCIS lesions; 81% of DCIS lesions contain PD-L1-positive TILs [159]. Moderate-diffuse TILs are more likely to be PD-L1-positive. PD-L1 TIL expression appears to be linked to DCIS phenotype: high PD-L1 expression (>50% cells) has only been identified in triple-negative DCIS, ER-negative lesions have PD-L1-positive TILs, and PD-L1-negative TILs were only identified in ER-positive luminal A DCIS [159].
A HER2 vaccine has shown promise in HER2-positive DCIS. Neoadjuvant treatment with the vaccine not only reduced the risk of residual disease at surgery, but eradicated HER2 expression in 50% of those patients with residual disease [160]. However, effectiveness of the vaccine was dependent on hormone receptor expression; only 6% of patients with ER-positive disease were free of residual disease at surgery compared with 40% of patients with ER-negative disease. The vaccine also altered disease phenotypes, with >40% of patients with initial ER/HER2-positive luminal B-type disease ending as ER-positive/HER2-negative luminal A [160].
A summary of ongoing clinical trials exploring HER2-targeted therapies in HER2-positive DCIS is provided in Table 1.
Conclusions
DCIS is biologically a heterogenous disease with variable outcomes, ranging from indolent lesions that will never become clinically significant to aggressive forms that recur as invasive breast cancer with potential to metastasize. In this context, pathologists should be conservative in diagnosing low-grade and low-volume DCIS on core needle biopsies. While progress towards personalized risk stratification and prediction of benefit from radiotherapy has been made, there is still a significant challenge in tailoring treatment to each individual patient. A substantial component in the risk–benefit equation is rather philosophical and patient-driven, as the acceptable risk of local recurrence or, more importantly, invasive recurrence or breast cancer mortality that one would tolerate is subjective per patient and multifactorial in nature. Risk tolerance preference applies to both ends of the biologic spectrum; on the one end, patients and healthcare providers need to decide when the risk is low enough to consider a surveillance trial or be treated with breast-conserving surgery alone, and on the other end, they need to decide when the risk is high enough to prompt more extensive treatment modalities, such as mastectomy with sentinel lymph node biopsy with or without systemic therapy.
Clinical, pathologic, biologic, and molecular markers, and genetic signatures have been helpful to a certain degree in the risk stratification and treatment-decision process. However, there is no simple, well-validated marker or group of variables for risk estimation to identify patients with an acceptably low risk to spare them surgical treatment and/or adjuvant therapies following breast-conserving surgery. The absolute benefit of these treatments should be determined by the magnitude of underlying baseline risk [161, 162]. There are tremendous focused research efforts to identify accurate risk stratification predictors of invasive recurrence and treatment response to radiotherapy and endocrine therapy, in addition to innovative treatment strategies, such as targeted therapies, immune modulators, and vaccines. Their results have the potential to bridge the persistent gap in DCIS evaluation and management, and therefore fulfill the promise of personalized care for patients with this prevalent and still challenging disease.
References
Howlader N, Noone AM, Krapcho M, et al. National Cancer Institute. SEER Cancer Statistics Review, 1975–2014 [based on November 2016 SEER data submission, posted to the SEER web site, April 2017]. https://seer.cancer.gov/csr/1975_2014/. Accessed 2018.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Levinsohn E, Altman M, Chagpar AB. Controversies regarding the diagnosis and management of ductal carcinoma in situ. Am Surg. 2018;84:1–6.
Lakhani SR, Ellis IO, Schnitt SJ et al., editors. WHO/IARC Classification of Tumours of the Breast. 4th ed, Vol 4. Lyon, France: World Health Organization (WHO), International Agency for Research on Cancer (IARC); 2012.
Bijker N, Donker M, Wesseling J, et al. Is DCIS breast cancer, and how do I treat it? Curr Treat Options Oncol. 2013;14:75–87.
Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–13.
Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1:888–96.
Stuart KE, Houssami N, Taylor R, et al. Long-term outcomes of ductal carcinoma in situ of the breast: a systematic review, meta-analysis and meta-regression analysis. BMC Cancer. 2015;15:890.
Sanders ME, Schuyler PA, Simpson JF, et al. Continued observation of the natural history of low-grade ductal carcinoma in situ reaffirms proclivity for local recurrence even after more than 30 years of follow-up. Mod Pathol. 2015;28:662–9.
Muggerud AA, Hallett M, Johnsen H, et al. Molecular diversity in ductal carcinoma in situ (DCIS) and early invasive breast cancer. Mol Oncol. 2010;4:357–68.
Perez AA, Rocha RM, Balabram D, et al. Immunohistochemical profile of high-grade ductal carcinoma in situ of the breast. Clinics (Sao Paulo). 2013;68:674–8.
Yang RL, Mick R, Lee K, et al. DCIS in BRCA1 and BRCA2 mutation carriers: prevalence, phenotype, and expression of oncodrivers C-MET and HER3. J Transl Med. 2015;13:335.
Zhou W, Jirström K, Amini RM, et al. Molecular subtypes in ductal carcinoma in situ of the breast and their relation to prognosis: a population-based cohort study. BMC Cancer. 2013;13:512.
Hwang ES, McLennan JL, Moore DH, et al. Ductal carcinoma in situ in BRCA mutation carriers. J Clin Oncol. 2007;25:642–7.
Bernardi D, Macaskill P, Pellegrini M, et al. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. Lancet Oncol. 2016;17:1105–13.
Brem RF, Lenihan MJ, Lieberman J, et al. Screening breast ultrasound: past, present, and future. Am J Roentgenol. 2015;204:234–40.
Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103:478–88.
Curigliano G, Burstein HJ, P Winer E, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700–12.
National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer. Version 3. 2018. https://www.nccn.org. Accessed 2018.
Donker M, Litière S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;31:4054–9.
Rakovitch E, Nofech-Mozes S, Hanna W, et al. HER2/neu and Ki-67 expression predict non-invasive recurrence following breast-conserving therapy for ductal carcinoma in situ. Br J Cancer. 2012;106:1160–5.
Holmes P, Lloyd J, Chervoneva I, et al. Prognostic markers and long-term outcomes in ductal carcinoma in situ of the breast treated with excision alone. Cancer. 2011;117:3650–7.
Wärnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. J Clin Oncol. 2014;32:3613–8.
Solin LJ, Gray R, Hughes LL, et al. Surgical excision without radiation for ductal carcinoma in situ of the breast: 12-year results from the ECOG-ACRIN E5194 Study. J Clin Oncol. 2015;33:3938–44.
Rakovitch E, Nofech-Mozes S, Hanna W, et al. Omitting radiation therapy after lumpectomy for pure DCIS does not reduce the risk of salvage mastectomy. Breast. 2018;37:181–6.
Halasz LM, Sreedhara M, Chen YH, et al. Improved outcomes of breast-conserving therapy for patients with ductal carcinoma in situ. Int J Radiat Oncol Biol Phys. 2012;82:e581–6.
Cancer Care Ontario. Breast Cancer Treatment Pathway Map. Version 2015. 11. https://www.cancercareontario.ca/sites/ccocancercare/files/assets/DPMBreastTreatment.pdf. Accessed 2018.
Wong JS, Kaelin CM, Troyan SL, et al. Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24:1031–6.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Correa C, McGale P, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–77.
Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–9.
EORTC Breast Cancer Cooperative Group, EORTC Radiotherapy Group, Bijker N, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853--a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24:3381–7.
McCormick B, Winter K, Hudis C, et al. RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol. 2015;33:709–15.
Julien JP, Bijker N, Fentiman IS, et al. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomised phase III trial 10853. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Lancet. 2000;355:528–33.
Kim T, Park HK, Lee KH, et al. Is radiotherapy necessary for intermediate risk ductal carcinoma in situ after breast conserving surgery? Springerplus. 2014;3:405.
Curigliano G, Disalvatore D, Esposito A, et al. Risk of subsequent in situ and invasive breast cancer in human epidermal growth factor receptor 2-positive ductal carcinoma in situ. Ann Oncol. 2015;26:682–7.
Rakovitch E, Nofech-Mozes S, Narod SA, et al. Can we select individuals with low risk ductal carcinoma in situ (DCIS)? A population-based outcomes analysis. Breast Cancer Res Treat. 2013;138:581–90.
Withrow DR, Morton LM, Curtis RE, et al. Radiotherapy for ductal carcinoma in situ and risk of second non-breast cancers. Breast Cancer Res Treat. 2017;166:299–306.
Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804–9.
Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–20.
Lalani N, Paszat L, Sutradhar R, et al. Long-term outcomes of hypofractionation versus conventional radiation therapy after breast-conserving surgery for ductal carcinoma in situ of the breast. Int J Radiat Oncol Biol Phys. 2014;90:1017–24.
Vicini F, Shah C, Ben Wilkinson J, et al. Should ductal carcinoma-in-situ (DCIS) be removed from the ASTRO consensus panel cautionary group for off-protocol use of accelerated partial breast irradiation (APBI)? A pooled analysis of outcomes for 300 patients with DCIS treated with APBI. Ann Surg Oncol. 2013;20:1275–81.
Forbes JF, Sestak I, Howell A, et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet. 2016;387:866–73.
Margolese RG, Cecchini RS, Julian TB, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 2016;387:849–56.
Guerrieri-Gonzaga A, Sestak I, Lazzeroni M, et al. Benefit of low-dose tamoxifen in a large observational cohort of high risk ER positive breast DCIS. Int J Cancer. 2016;139:2127–34.
Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012;30:1268–73.
Ganz PA, Cecchini RS, Julian TB, et al. Patient-reported outcomes with anastrozole versus tamoxifen for postmenopausal patients with ductal carcinoma in situ treated with lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 2016;387:857–65.
Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94:1546–54.
Groen EJ, Elshof LE, Visser LL, et al. Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS). Breast. 2017;31:274–83.
Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005.
Maxwell AJ, Clements K, Hilton B, et al. Risk factors for the development of invasive cancer in unresected ductal carcinoma in situ. Eur J Surg Oncol. 2018;44:429–35.
Ryser MD, Worni M, Turner EL, et al. Outcomes of active surveillance for ductal carcinoma in situ: a computational risk analysis. J Natl Cancer Inst. 2015;108:djv372.
Chavez de Paz Villanueva C, Bonev V, Senthil M, et al. Factors associated with underestimation of invasive cancer in patients with ductal carcinoma in situ: Precautions for active surveillance. JAMA Surg. 2017;152:1007–14.
Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Ductal Carcinoma In Situ. J Clin Oncol. 2016;34:4040–6.
College of American Pathologists (CAP). Protocol for the examination of specimens from patients with ductal carcinoma in situ (DCIS) of the breast. Version: Breast DCIS 4.1.0.0. Protocol Posting Date: January 2018. http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/cp-breast-dcis-18protocol-4100.pdf. Accessed 2018.
Shamliyan T, Wang SY, Virnig BA, et al. Association between patient and tumor characteristics with clinical outcomes in women with ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010:121.
Wang SY, Shamliyan T, Virnig BA, et al. Tumor characteristics as predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Breast Cancer Res Treat. 2011;127:1–14.
Zhang X, Dai H, Liu B, et al. Predictors for local invasive recurrence of ductal carcinoma in situ of the breast: a meta-analysis. Eur J Cancer Prev. 2016;25:19–28.
Pruneri G, Lazzeroni M, Bagnardi V, et al. The prevalence and clinical relevance of tumor-infiltrating lymphocytes (TILs) in ductal carcinoma in situ of the breast. Ann Oncol. 2017;28:321–8.
Campbell MJ, Baehner F, O’Meara T, et al. Characterizing the immune microenvironment in high-risk ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2017;161:17–28.
Knopfelmacher A, Fox J, Lo Y, et al. Correlation of histopathologic features of ductal carcinoma in situ of the breast with the oncotype DX DCIS score. Mod Pathol. 2015;28:1167–73.
Cao Y, Paner GP, Kahn LB, et al. Noninvasive carcinoma of the breast: angiogenesis and cell proliferation. Arch Pathol Lab Med. 2004;128:893–6.
Guidi AJ, Fischer L, Harris JR, et al. Microvessel density and distribution in ductal carcinoma in situ of the breast. J Natl Cancer Inst. 1994;86:614–9.
Engels K, Fox SB, Whitehouse RM, et al. Distinct angiogenic patterns are associated with high-grade in situ ductal carcinomas of the breast. J Pathol. 1997;181:207–12.
Zolota V, Gerokosta A, Melachrinou M, et al. Microvessel density, proliferating activity, p53 and bcl-2 expression in in situ ductal carcinoma of the breast. Anticancer Res. 1999;19:3269–74.
Teo NB, Shoker BS, Jarvis C, et al. Angiogenesis and invasive recurrence in ductal carcinoma in situ of the breast. Eur J Cancer. 2003;39:38–44.
Adler EH, Sunkara JL, Patchefsky AS, et al. Predictors of disease progression in ductal carcinoma in situ of the breast and vascular patterns. Hum Pathol. 2012;43:550–6.
Onega T, Weaver DL, Frederick PD, et al. The diagnostic challenge of low-grade ductal carcinoma in situ. Eur J Cancer. 2017;80:39–47.
Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996;77:2267–74.
Silverstein MJ. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg. 2003;186:337–43.
Silverstein MJ, Lagios MD. Choosing treatment for patients with ductal carcinoma in situ: fine tuning the University of Southern California/Van Nuys Prognostic Index. J Natl Cancer Inst Monogr. 2010;2010:193–6.
Kelley L, Silverstein M, Guerra L. Analyzing the risk of recurrence after mastectomy for DCIS: a new use for the USC/Van Nuys Prognostic Index. Ann Surg Oncol. 2011;18:459–62.
Boland GP, Chan KC, Knox WF, et al. Value of the Van Nuys Prognostic Index in prediction of recurrence of ductal carcinoma in situ after breast-conserving surgery. Br J Surg. 2003;90:426–32.
Cornfield DB, Palazzo JP, Schwartz GF, et al. The prognostic significance of multiple morphologic features and biologic markers in ductal carcinoma in situ of the breast: a study of a large cohort of patients treated with surgery alone. Cancer. 2004;100:2317–27.
Asjoe FT, Altintas S, Huizing MT, et al. The value of the Van Nuys Prognostic Index in ductal carcinoma in situ of the breast: a retrospective analysis. Breast J. 2007;13:359–67.
Di Saverio S, Catena F, Santini D, et al. 259 patients with DCIS of the breast applying USC/Van Nuys Prognostic Index: a retrospective review with long term follow up. Breast Cancer Res Treat. 2008;109:405–16.
Gilleard O, Goodman A, Cooper M, et al. The significance of the Van Nuys Prognostic Index in the management of ductal carcinoma in situ. World J Surg Oncol. 2008;6:61.
Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol. 2010;28:3762–9.
Yi M, Meric-Bernstam F, Kuerer HM, et al. Evaluation of a breast cancer nomogram for predicting risk of ipsilateral breast tumor recurrences in patients with ductal carcinoma in situ after local excision. J Clin Oncol. 2012;30:600–7.
Sweldens C, Peeters S, van Limbergen E, et al. Local relapse after breast-conserving therapy for ductal carcinoma in situ: a European single-center experience and external validation of the Memorial Sloan-Kettering Cancer Center DCIS nomogram. Cancer J. 2014;20:1–7.
Collins LC, Achacoso N, Haque R, et al. Risk prediction for local breast cancer recurrence among women with DCIS treated in a community practice: A nested, case-control study. Ann Surg Oncol. 2015;22:S502–8.
Punglia RS, Jiang W, Lipsitz SR, et al. Clinical risk score to predict likelihood of recurrence after ductal carcinoma in situ treated with breast-conserving surgery. Breast Cancer Res Treat. 2018;167:751–9.
Van Zee KJ, Subhedar P, Olcese C, et al. Relationship between margin width and recurrence of ductal carcinoma in situ: Analysis of 2996 women treated with breast-conserving surgery for 30 years. Ann Surg. 2015;262:623–31.
Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer. 1999;86:429–38.
Marinovich ML, Azizi L, Macaskill P, et al. The association of surgical margins and local recurrence in women with ductal carcinoma in situ treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol. 2016;23:3811–21.
Tang SS, Kaptanis S, Haddow JB, et al. Current margin practice and effect on re-excision rates following the publication of the SSO-ASTRO consensus and ABS consensus guidelines: a national prospective study of 2858 women undergoing breast-conserving therapy in the UK and Ireland. Eur J Cancer. 2017;84:315–24.
Kuerer HM, Smith BD, Chavez-MacGregor M, et al. DCIS margins and breast conservation: MD Anderson Cancer Center multidisciplinary practice guidelines and outcomes. J Cancer. 2017;8:2653–62.
Tadros AB, Smith BD, Shen Y, et al. Ductal carcinoma in situ and margins <2 mm: contemporary outcomes with breast conservation. Ann Surg. 2019;269:150–7.
Faverly DR, Burgers L, Bult P, et al. Three dimensional imaging of mammary ductal carcinoma in situ: clinical implications. Semin Diagn Pathol. 1994;11:193–8.
Merrill AL, Tang R, Plichta JK, et al. Should new “no ink on tumor” lumpectomy margin guidelines be applied to ductal carcinoma in situ (DCIS)? A retrospective review using shaved cavity margins. Ann Surg Oncol. 2016;23:3453–8.
Hodi Z, Ellis IO, Elston CW, et al. Comparison of margin assessment by radial and shave sections in wide local excision specimens for invasive carcinoma of the breast. Histopathology. 2010;56:573–80.
Fitzsullivan E, Lari SA, Smith B, et al. Incidence and consequence of close margins in patients with ductal carcinoma-in situ treated with mastectomy: is further therapy warranted? Ann Surg Oncol. 2013;20:4103–12.
Klein J, Kong I, Paszat L, et al. Close or positive resection margins are not associated with an increased risk of chest wall recurrence in women with DCIS treated by mastectomy: a population-based analysis. Springerplus. 2015;4:335.
Dooley WC, Parker J. Understanding the mechanisms creating false positive lumpectomy margins. Am J Surg. 2005;190:606–8.
Molina MA, Snell S, Franceschi D, et al. Breast specimen orientation. Ann Surg Oncol. 2009;16:285–8.
Singh M, Singh G, Hogan KT, et al. The effect of intraoperative specimen inking on lumpectomy re-excision rates. World J Surg Oncol. 2010;8:4.
Wright MJ, Park J, Fey JV, et al. Perpendicular inked versus tangential shaved margins in breast-conserving surgery: does the method matter? J Am Coll Surg. 2007;204:541–9.
Chagpar AB, Killelea BK, Tsangaris TN, et al. A randomized, controlled trial of cavity shave margins in breast cancer. N Engl J Med. 2015;373:503–10.
Cao D, Lin C, Woo SH, et al. Separate cavity margin sampling at the time of initial breast lumpectomy significantly reduces the need for reexcisions. Am J Surg Pathol. 2005;29:1625–32.
Sigal-Zafrani B, Lewis JS, Clough KB, et al. Histological margin assessment for breast ductal carcinoma in situ: precision and implications. Mod Pathol. 2004;17:81–8.
Neuschatz AC, DiPetrillo T, Steinhoff M, et al. The value of breast lumpectomy margin assessment as a predictor of residual tumor burden in ductal carcinoma in situ of the breast. Cancer. 2002;94:1917–24.
Lyman GH, Somerfield MR, Bosserman LD, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society Of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2017;35:561–4.
Ansari B, Ogston SA, Purdie CA, et al. Meta-analysis of sentinel node biopsy in ductal carcinoma in situ of the breast. Br J Surg. 2008;95:547–54.
Tunon-de-Lara C, Chauvet MP, Baranzelli MC, et al. The role of sentinel lymph node biopsy and factors associated with invasion in extensive DCIS of the breast treated by mastectomy: the Cinnamome Prospective Multicenter Study. Ann Surg Oncol. 2015;22:3853–60.
Han JS, Molberg KH, Sarode V. Predictors of invasion and axillary lymph node metastasis in patients with a core biopsy diagnosis of ductal carcinoma in situ: an analysis of 255 cases. Breast J. 2011;17:223–9.
Trentin C, Dominelli V, Maisonneuve P, et al. Predictors of invasive breast cancer and lymph node involvement in ductal carcinoma in situ initially diagnosed by vacuum-assisted breast biopsy: experience of 733 cases. Breast. 2012;21:635–40.
Meretoja TJ, Heikkila PS, Salmenkivi K, et al. Outcome of patients with ductal carcinoma in situ and sentinel node biopsy. Ann Surg Oncol. 2012;19:2345–51.
Tada K, Ogiya A, Kimura K, et al. Ductal carcinoma in situ and sentinel lymph node metastasis in breast cancer. World J Surg Oncol. 2010;8:6.
Tunon-de-Lara C, Giard S, Buttarelli M, et al. Sentinel node procedure is warranted in ductal carcinoma in situ with high risk of occult invasive carcinoma and microinvasive carcinoma treated by mastectomy. Breast J. 2008;14:135–40.
Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014;32:1365–83.
Pilewskie M, Karsten M, Radosa J, et al. Is sentinel lymph node biopsy indicated at completion mastectomy for ductal carcinoma in situ? Ann Surg Oncol. 2016;23:2229–34.
Pilewskie M, Stempel M, Rosenfeld H, et al. Do LORIS trial eligibility criteria identify a ductal carcinoma in situ patient population at low risk of upgrade to invasive carcinoma? Ann Surg Oncol. 2016;23:3487–93.
Lari SA, Kuerer HM. Biological markers in DCIS and risk of breast recurrence: a systematic review. J Cancer. 2011;2:232–61.
Doebar SC, van den Broek EC, Koppert LB, et al. Extent of ductal carcinoma in situ according to breast cancer subtypes: a population-based cohort study. Breast Cancer Res Treat. 2016;158:179–87.
Zhou W, Johansson C, Jirström K, et al. A comparison of tumor biology in primary ductal carcinoma in situ recurring as invasive carcinoma versus a new in situ. Int J Breast Cancer. 2013;2013:582134.
Molinaro AM, Sison JD, Ljung BM, et al. Risk prediction for local versus regional/metastatic tumors after initial ductal carcinoma in situ diagnosis treated by lumpectomy. Breast Cancer Res Treat. 2016;157:351–61.
Provenzano E, Hopper JL, Giles GG, et al. Biological markers that predict clinical recurrence in ductal carcinoma in situ of the breast. Eur J Cancer. 2003;39:622–30.
Kerlikowske K, Molinaro AM, Gauthier ML, et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst. 2010;102:627–37.
Eng-Wong J, Costantino JP, Swain SM. The impact of systemic therapy following ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010:200–3.
Ringberg A, Anagnostaki L, Anderson H, et al. Cell biological factors in ductal carcinoma in situ (DCIS) of the breast-relationship to ipsilateral local recurrence and histopathological characteristics. Eur J Cancer. 2001;37:1514–22.
Yu Q, Niu Y, Liu N, et al. Expression of androgen receptor in breast cancer and its significance as a prognostic factor. Ann Oncol. 2011;22:1288–94.
Gonzalez LO, Corte MD, Junquera S, et al. Expression of androgen receptor and two androgen-induced proteins (apolipoprotein D and pepsinogen C) in ductal carcinoma in situ of the breast. Histopathology. 2007;50:866–74.
Hanley K, Wang J, Bourne P, et al. Lack of expression of androgen receptor may play a critical role in transformation from in situ to invasive basal subtype of high-grade ductal carcinoma of the breast. Hum Pathol. 2008;39:386–92.
Ravaioli S, Tumedei MM, Foca F, et al. Androgen and oestrogen receptors as potential prognostic markers for patients with ductal carcinoma in situ treated with surgery and radiotherapy. Int J Exp Pathol. 2017;98:289–95.
Tumedei MM, Silvestrini R, Ravaioli S, et al. Role of androgen and estrogen receptors as prognostic and potential predictive markers of ductal carcinoma in situ of the breast. Int J Biol Markers. 2015;30:e425–8.
Davis JE, Nemesure B, Mehmood S, et al. Her2 and Ki67 biomarkers predict recurrence of ductal carcinoma in situ. Appl Immunohistochem Mol Morphol. 2016;24:20–5.
Poulakaki N, Makris GM, Papanota AM, et al. Ki-67 expression as a factor predicting recurrence of ductal carcinoma in situ of the breast: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:157–67.e6.
Kim JY, Park K, Kang G, et al. Predictors of recurrent ductal carcinoma in situ after breast-conserving surgery. J Breast Cancer. 2016;19:185–90.
Bosch DE, Kilgore MR, Schmidt RA, et al. Comparison of proliferation markers Ki67 and phosphohistone-h3 (pHH3) in breast ductal carcinoma in situ. Appl Immunohistochem Mol Morphol. 2017;25:543–7.
Han K, Nofech-Mozes S, Narod S, et al. Expression of HER2neu in ductal carcinoma in situ is associated with local recurrence. Clin Oncol (R Coll Radiol). 2012;24:183–9.
Witkiewicz AK, Cox DW, Rivadeneira D, et al. The retinoblastoma tumor suppressor pathway modulates the invasiveness of ErbB2-positive breast cancer. Oncogene. 2014;33:3980–91.
Borgquist S, Zhou W, Jirström K, et al. The prognostic role of HER2 expression in ductal breast carcinoma in situ (DCIS); a population-based cohort study. BMC Cancer. 2015;15:468.
Williams KE, Barnes NL, Cramer A, et al. Molecular phenotypes of DCIS predict overall and invasive recurrence. Ann Oncol. 2015;26:1019–25.
Wärnberg F, Garmo H, Folkvaljon Y, et al. A validation of DCIS biological risk profile in a randomised study for radiation therapy with 20 year follow-up (SweDCIS). Cancer Res. 2018;78:Abstract GS5–08.
Bremer T, Linke SP, Whitworth PW, et al. A multi-marker prognostic to assess risk of invasive recurrence in DCIS patients. J Clin Oncol. 2016;34:Abstract 1019.
Pang JB, Savas P, Fellowes AP, et al. Breast ductal carcinoma in situ carry mutational driver events representative of invasive breast cancer. Mod Pathol. 2017;30:952–63.
Buckley N, Boyle D, McArt D, et al. Molecular classification of non-invasive breast lesions for personalised therapy and chemoprevention. Oncotarget. 2015;6:43244–54.
Hieken TJ, Cheregi J, Farolan M, et al. Predicting relapse in ductal carcinoma in situ patients: an analysis of biologic markers with long-term follow-up. Am J Surg. 2007;194:504–6.
de Roos MA, de Bock GH, de Vries J, et al. P53 overexpression is a predictor of local recurrence after treatment for both in situ and invasive ductal carcinoma of the breast. J Surg Res. 2007;140:109–14.
Generali D, Buffa FM, Deb S, et al. COX-2 expression is predictive for early relapse and aromatase inhibitor resistance in patients with ductal carcinoma in situ of the breast, and is a target for treatment. Br J Cancer. 2014;111:46–54.
Bundred NJ, Cramer A, Morris J, et al. Cyclooxygenase-2 inhibition does not improve the reduction in ductal carcinoma in situ proliferation with aromatase inhibitor therapy: results of the ERISAC randomized placebo-controlled trial. Clin Cancer Res. 2010;16:1605–12.
Jiang Y, Prabakaran I, Wan F, et al. Vav2 protein overexpression marks and may predict the aggressive subtype of ductal carcinoma in situ. Biomark Res. 2014;2:22.
Theurillat JP, Ingold F, Frei C, et al. NY-ESO-1 protein expression in primary breast carcinoma and metastases: correlation with CD8+T-cell and CD79a+plasmacytic/B-cell infiltration. Int J Cancer. 2007;120:2411–7.
Jäger E, Chen YT, Drijfhout JW, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187:265–70.
Ademuyiwa FO, Bshara W, Attwood K, et al. NY-ESO-1 cancer testis antigen demonstrates high immunogenicity in triple negative breast cancer. PLoS One. 2012;7:e38783.
Coombes RC, Caballero OL, Shousha S, et al. NY-ESO-1 expression in DCIS: a new predictor of good prognosis. Oncoscience. 2017;4:33–40.
Altintas S, Lambein K, Huizing MT, et al. Prognostic significance of oncogenic markers in ductal carcinoma in situ of the breast: a clinicopathologic study. Breast J. 2009;15:120–32.
Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105:701–10.
Rakovitch E, Nofech-Mozes S, Hanna W, et al. A population-based validation study of the DCIS Score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat. 2015;152:389–98.
Lin CY, Mooney K, Choy W, et al. Will oncotype DX DCIS testing guide therapy? A single-institution correlation of oncotype DX DCIS results with histopathologic findings and clinical management decisions. Mod Pathol. 2018;31:562–8.
Alvarado M, Carter DL, Guenther JM, et al. The impact of genomic testing on the recommendation for radiation therapy in patients with ductal carcinoma in situ: a prospective clinical utility assessment of the 12-gene DCIS score™ result. J Surg Oncol. 2015;111:935–40.
Shumway DA, McLeod CM, Morrow M, et al. Patient experiences and clinician views on the role of radiation therapy for ductal carcinoma in situ. Int J Radiat Oncol Biol Phys. 2018;100:1237–45.
ClinicalTrials.gov. Evaluation of the DCIS Score for Decisions on Radiotherapy in Patients With Low/Intermediate Risk DCIS (DUCHESS). ClinicalTrials.gov Identifier: NCT02766881. https://clinicaltrials.gov/ct2/show/NCT02766881. Accessed 2018.
Raldow AC, Sher D, Chen AB, et al. Cost effectiveness of the Oncotype DX DCIS score for guiding treatment of patients with ductal carcinoma in situ. J Clin Oncol. 2016;34:3963–8.
Alvarado M, Lucci A, Manders J. Best practices for multidisciplinary integration of a DCIS genomic assay into clinical practice. J Surg Oncol. 2017;116:1016–20.
Gorringe KL, Fox SB. Ductal carcinoma in situ biology, biomarkers, and diagnosis. Front Oncol. 2017;7:248.
Hannafon BN, Ding WQ. miRNAs as biomarkers for predicting the progression of ductal carcinoma in situ. Am J Pathol. 2018;188:542–9.
Wiechmann L, Kuerer HM. The molecular journey from ductal carcinoma in situ to invasive breast cancer. Cancer. 2008;112:2130–42.
Morita M, Yamaguchi R, Tanaka M, et al. CD8(+) tumor-infiltrating lymphocytes contribute to spontaneous “healing” in HER2-positive ductal carcinoma in situ. Cancer Med. 2016;5:1607–18.
Thompson E, Taube JM, Elwood H, et al. The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol. 2016;29:249–58.
Sharma A, Koldovsky U, Xu S, et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer. 2012;118:4354–62.
Shah C, Wobb J, Manyam B, et al. Management of ductal carcinoma in situ of the breast: a review. JAMA Oncol. 2016;2:1083–8.
Duffy SW, Dibden A, Michalopoulos D, et al. Screen detection of ductal carcinoma in situ and subsequent incidence of invasive interval breast cancers: a retrospective population-based study. Lancet Oncol. 2016;17:109–14.
Acknowledgements
The support for third-party editorial support for this manuscript, furnished by Helen Keyworth, PhD, of Health Interactions, was provided by a Departmental Grant from Sunnybrook Health Sciences Center, Toronto, Ontario, Canada and F Hoffmann-La Roche Ltd, Basel, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hanna, W.M., Parra-Herran, C., Lu, FI. et al. Ductal carcinoma in situ of the breast: an update for the pathologist in the era of individualized risk assessment and tailored therapies. Mod Pathol 32, 896–915 (2019). https://doi.org/10.1038/s41379-019-0204-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0204-1
This article is cited by
-
A clinical radiomics nomogram preoperatively to predict ductal carcinoma in situ with microinvasion in women with biopsy-confirmed ductal carcinoma in situ: a preliminary study
BMC Medical Imaging (2023)
-
Diagnosis of ductal carcinoma in situ in an era of de-escalation of therapy
Modern Pathology (2021)
-
Single-cell evaluation reveals shifts in the tumor-immune niches that shape and maintain aggressive lesions in the breast
Nature Communications (2021)
-
Immune microenvironment in different molecular subtypes of ductal breast carcinoma
Breast Cancer Research and Treatment (2021)
-
CDH2/N-cadherin and early diagnosis of invasion in patients with ductal carcinoma in situ
Breast Cancer Research and Treatment (2020)