Abstract
The transport of proteins across the nuclear membrane is a highly regulated process, essential for the cell function. This transport is actively mediated by members of the karyopherin family, termed importins, or exportins, depending on the direction of transport. These proteins play an active part in tumorigenesis, through aberrant localization of their cargoes, which include oncogenes, tumor-suppressor genes and mediators of key signal transduction pathways. Overexpression of importins and exportins is reported in many malignancies, with implications in cell growth and viability, differentiation, drug resistance, and tumor microenvironment. Given their broad significance across tumors and pathways, much effort is being put to develop specific inhibitors as a novel anticancer therapeutics. Already, selinexor, a specific inhibitor of exportin-1 (XPO1), is approved for clinical use. This review will focus on the role of importins and exportins in hematological malignancies. We will discuss current preclinical and clinical data on importins and exportins, and demonstrate how our growing understanding of their functions has identified new therapeutic targets.
Similar content being viewed by others
Introduction
The nuclear membrane forms the barrier between protein transcription in the nucleus and protein translation in the cytoplasm, which is key feature of the eukaryotic cell. This separation allows for controlled transport of proteins in and out of the nucleus, which tightly regulates many cell functions. The transport of proteins across the nuclear membrane is mediated by nuclear transport receptors of the karyopherin family, which includes the importin (IPO) α and IPOβ subfamilies. IPOβ subfamily is further divided to IPOs and exportins depending on the direction of transport, in or out of the nucleus, respectively. Dysregulation of the nuclear import and export machinery is common in cancer, resulting in aberrant localization of various proteins, including tumor-suppressor genes and oncogenes that tumorigenesis [1]. Overexpression of IPOs and exportins is reported in numerous malignancies, and is often correlated with poor prognosis and a more aggressive tumor phenotype
This review will focus on the significance of IPOs and exportins in the pathogenesis of various hematological malignancies. We will highlight how dysregulation of nuclear transport activates key signal transduction pathways with implications in cell proliferation, apoptosis, and drug resistance. We will review the current preclinical studies with specific inhibitors to IPOs and exportins and demonstrate how these findings translate to clinical trials.
Nuclear transport machinery
The nuclear transport machinery has three primary components: (1) the nuclear pore complex (NPC), (2) the nuclear transport receptors, and (3) small Ras-like GTPase RAN that facilitates nuclear protein import and export by providing a gradient across the nuclear membrane and energy for the transport. The NPC is a basket-like structure composed of multiple copies of up to 50 different proteins termed nucleoporins (Nups). Nups are subdivided into scaffold Nups that form a cylindrical channel about 9–10 nm in diameter and 15 nm long [2], and phenylalanine-glycine Nups that anchor the scaffold Nups to the nuclear membrane and form a selective barrier for nuclear transport [3]. Small (<10 nm, MW < 300) molecules passively traverse this complex, but larger cargo requires active, energy dependent, shuttling mediated by nuclear transport receptors. The majority of nuclear transport receptors are members of the karyopherin family of proteins.
Ten human IPOs are known: IPOβ1, transportin (TNPO) 1 and 2, IPO4, 5, 7 8, 9, 11 and 12. The six human exportins are: Chromosome region maintenance 1 (CRM1)/exportin-1 (XPO1), cellular apoptosis susceptibility (CAS/CSEIL), exportin (XPO) 5, 6 and T, and RanBP17 (Table 1). Certain family members, such as IPO13 facilitate bidirectional transport, while the remaining family members have unidirectional transport functions. All IPOβ family members share a similar tandem Huntingtin, Elongation factor 3, protein phosphatase 2A, and yeast kinase TOR1 (HEAT) repeats. One HEAT repeat comprises two α-helices (of 10–20 residues each) that are linked by a short intra-repeat loop. The tandem HEAT repeat fold is inherently flexible and contribute substantially to Ran-controlled cargo recognition and release. IPOβ proteins bind and shuttle their cargo directly, or use IPOα as an adapter protein to bind cargo. The IPOα family includes seven members in humans and functions mainly as an adapter for the carrier protein. Aside from the IPOβ binding (IBB) domain, IPOα bears an armadillo motif that recognize the nuclear localizing signal (NLS) and C-terminal region that binds to the nuclear export factor of IPOα [4].
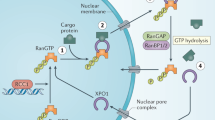
The binding of IPOα to NLS-cargo unmasks the IBB domain for IBB to facilitate nuclear import (Fig. 1). Thus, the trimeric complex is needed for successful transport, and eliminates futile transport of empty IPOα/β complex [5]. Next, the IPOβ–IPOα-cargo complex translocates into the nucleus through the NPC. The final key component that allows for the release of the cargo in the nucleus, provide energy for the transport and maintain polarity is the Ras-related small GTPase Ran. In the nucleus abundant GTPase Ran binds to IPOβ, in two regions located between HEAT repeats 1–4 and 12–15, partially overlapping with the IBB domain. Binding of RanGTP acts allosterically on IPOβ inducing conformational changes releasing IPOβ from IPOα. In the cytoplasm, Ran is mainly bound to GDP, thus forming a gradient that directs the import of proteins into the nucleus. The regulator of chromosome condensation 1 (RCC1) reforms RanGTP from RanGDP in the nucleus and plays a critical part in maintaining the gradient across the nuclear membrane [5].
The importin α/β-cargo complex translocate into the nucleus through the NPC. In the nucleus, binding of abundant RanGTP mediates the release of the cargo. Gray text: hematological malignancies in which the nuclear transport of the specific cargo is shown to be significant. Star marks the binding site of the specified inhibitor in red. NLS nuclear localizing signal, NPC nuclear pore complex, DLBCL diffuse large B-cell lymphoma, APL acute promyelocytic leukemia, MM multiple myeloma, NHL non-Hodgkin lymphoma, CLL chronic lymphocytic leukemia.
Cargo proteins are recognized by a nuclear localizing sequence or nuclear export sequence (NES). The classical NLS of the SV40-T antigen harbors a short characteristic ~7 basic residues (PKKKRKV) rich in lysine and arginine residues. The NLS of nucleoplasmin is the prototype of the bipartite signal with a small cluster of basic residues positioned 10–12 residues N-terminal to a monopartite-like sequence [6]. Further study revealed more noncanonical NLS, including the 38 residue M9 sequence of hnRNP A1, PTHrP and a proline-tyrosine (PY-NLS) consensus sequence [7]. Each IPO can import a variety of different cargo proteins using different binding sites within the same IPO. A recent study has utilized proximity ligation mass spectrometry based on the BioID system to map the various cargo of each IPO [3]. This allows a better understanding of the specificity and redundancy in nuclear transport by the various IPOs. While a set of distinct cargoes can be identified for each IPO, redundancy exists. Some IPOs such as IPO4 and IPO5 show a significant overlap. Remarkably, functionally related proteins involved in similar biological processes cluster with a specific IPO. In addition, different IPOs bind to different FG-Nups, promoting a highly specific nuclear import. Together the findings suggest a link between function and a designated import cascade [3].
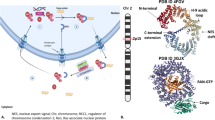
The classic nuclear export signal consists of a leucin-rich stretch. CRM1/XPO1, the best characterized exportin, has a ring-like shape with a hydrophobic grove formed by HEAT repeats 11 and 12 that recognize and bind leucin-rich NES on cargo proteins [8]. Similar to IPOs, nuclear export by exportin are dependent on RanGTP gradient. However, as opposed to IPOs, exportins recruit their cargo at high RanGTP levels in the nucleus. RanGTP binds XPO1 to facilitate its active transport through the NPC (Fig. 2). In the cytoplasm, RanBP1 and Ran GTPase-activating protein 1 (RanGAP1) mediate cargo release and RanGTP hydrolysis to RanGDP [9].
In the cytoplasm and Ran GTPase-activating protein 1 (RanGAP1) mediates cargo release and RanGTP hydrolysis to RanGDP. The regulator of chromosome condensation 1 (RCC1) reforms RanGTP from RanGDP in the nucleus to maintain the gradient across the nuclear membrane. Star marks the binding site of the specified inhibitor in red. NPC nuclear pore complex.
Expression and regulation of exportins in hematological malignancies
XPO1 is upregulated in many solid tumors and hematological malignancies and its expression is associated with adverse effect on prognosis [10,11,12]. For example, myeloma cells have a high XPO1 expression compared to normal plasma cells, and its expression has been shown to increase with progression, to be associated with increased lytic bone lesions and shorter survival [13]. A study that evaluated 511 newly diagnosed AML patients also found high expression of XPO1, that is associated with short survival in multivariate analysis [14]. Similar findings were reported in diffuse large B-cell lymphoma (DLBCL) [15], chronic lymphocytic leukemia (CLL) [16], and T cell lymphoma [17, 18]. In mantle cell lymphoma (MCL), higher XPO1 expression at diagnosis is associated with a poorer prognosis, with a median overall survival of 3.2 years in the low expression XPO1 cases vs 1.9 years in the high expression XPO1 cases [18].
The regulation of exportin expression is not yet completely understood, and most studies fail to demonstrate a cytogenetic or molecular mutation leading to XPO1 overexpression. On the other hand, hallmark oncogenes such as c-MYC and BCR-ABL directly enhance transcription of XPO1, while p53 negatively regulates XPO1 levels by repressing basal expression and attenuating its induction by c-MYC [19, 20]. Interestingly, the interplay between XPO1 and such oncogenes creates a vicious cycle as XPO1 enhances their activity and in return they support XPO1 expression. Although not common, genetic alterations might also contribute to XPO1 expression. A report in T-ALL discovered a cryptic translocation involving XPO1 and MLL10 with deregulation of HOXA gene locus expression [21]. Copy number gains in the XPO1 locus also occur in primary mediastinal B-cell lymphoma [22].
Finally, several mutations in XPO1 are identified in hematological malignancies. Mutation E571K in XPO1 are found in up 30% of classical Hodgkin disease and primary mediastinal lymphoma. However, the significance of the mutation is still not clear and no correlation with PFS or OS is noted [23, 24]. Missense mutations in XPO1 are reported in a small subset of CLL patients with correlation to unmutated IGHV status, however it is not associated with adverse prognosis [25].
Pro-tumorigenic pathways involving exportins
As mentioned above, exportins recognize and bind NES-bearing cargoes in the high RanGTP environment of the nucleus. Among XPO1’s cargo are tumor-suppressor proteins (e.g., p53, Rb, p21, p27, APC, and FOXO), mediators of key signal transduction pathways (e.g., IkB), proto-oncogenes (e.g., survivin, BCR-ABL, BRCA1, and Fbw7) and the drug target topoisomerase (Topo) IIα [10, 26].
For example, p53 subcellular localization is tightly regulated in normal cells and governs its function. While it accumulates in the cytoplasm during the G1 phase of cell cycle, p53 enters the nucleus during the G1/S phase transition [27]. Nuclear exclusion of p53 is observed in many tumors and is mediated by XPO1 [28]. Inhibition of XPO1 in AML cells also induces nuclear accumulation of p53, concomitant with decreased growth and viability and induction of differentiation. Accordingly, primary AML cells with defective p53 are much less sensitive to XPO1 inhibition, suggesting the anti-tumorigenic effect of XPO1 is p53 dependent [14]. Similar findings are reported in CLL, multiple myeloma and MCL [13, 17, 18].
High XPO1 expression also supports NF-kB signaling, a key feature in many hematological malignancies, including non-Hodgkin lymphoma, CLL and multiple myeloma. XPO1 mediates the nuclear export of IkB, a key inhibitor of NF-kB transcriptional activity [16, 29, 30]. High expression of XPO1 increases the efflux of IkB, promoting its proteasomal degradation in the cytoplasm, with resulting higher NF-kB activity [31].
Another XPO1 cargo with wide implications in cancer is Topo IIa. Topo IIa nuclear export, mediated by XPO1, does not allow topo II inhibitors such as doxorubicin to induce Topo II/DNA cleavable complexes and resulting apoptosis. XPO1 overexpression thus promotes resistance to Topo inhibitors [32].
Others cargoes of XPO1 are more tumor specific. For example, in AML, the common nucleophosmin 1 (NPM1) mutation promotes the cytoplasmic localization of NPM1 by introducing an XPO1-responsive NES and disrupts the nuclear localization signal [33]. Nuclear re-localization of NPM1 either by genetic manipulation or by inhibiting XPO1 results in loss of HOX genes expression and differentiation of AML cells [34]. AML blasts with cytoplasmic NPM1 are most responsive to XPO1 inhibition [35]. Other examples of tumor- specific cargoes are nuclear export of cyclin D1 mRNA in MCL, with decreased cyclin D1 levels upon inhibition of XPO1 [36, 37], and BCR-ABL in chronic myeloid leukemia (CML), as elaborated below. Finally, nuclear export of signal transducer and activator of transcription (STAT)6 by XPO1 in primary B-cell mediastinal lymphoma augments the Janus Kinase (JAK)/STAT6 signal transduction pathway [38].
In comparison to the wide range of cargoes of XPO1, other exportins show a more restricted range of cargoes. Exportin 2 (XPO2), also called CAS, is a highly interesting protein, as its main cargoes are IPOα [39]. Thus, in essence it regulates nuclear import, and might be target for nuclear import inhibition. High levels of XPO2 are found in many cancer cells lines, including leukemia [40]. However, further studies are needed to clarify its role in cancer biology. The significance of other exportins in cancer is beyond the scope of this review, and readers are referred to the comprehensive review by Cagatay et al. [1].
Selective inhibitors of nuclear export (SINE)
Given the wide dysregulation of nuclear transport in cancer and their function at the crossroad of many critical signal transduction pathways, targeting exportins has been the focus of many studies in cancer.
Several small molecule inhibitors of XPO1 have been identified. Leptomycin B, initially used as an antifungal agent, was the first XPO1 inhibitor to be reported. Leptomycin B binds to cysteine residue 528 in the hydrostatic grove of XPO1. Further chemical reaction driven by XPO1 and by RanBP1 optimizes the leptomycin B-XPO1 interaction, making it irreversible and potentiates the inactivation of XPO1 by spatial inhibition of NES binding [41, 42]. While numerous studies utilized leptomycin B to inhibit XPO1 in cell lines, allowing the discovery of many of XPO1 cargoes, the molecule is not suitable for clinical use. A single trial with leptomycin B in patients with refractory advanced solid tumors failed to show any efficacy, while marked toxicity of anorexia and malaise were reported [43]. The toxicity observed with leptomycin B is thought to be related to its irreversible binding and inhibition of XPO1, which cripples nuclear export in normal cells as well [44]. As a result of the experiences with Leptomycin B, medicinal chemistry effort focused on developing selective, potent, and reversible inhibitors of XPO1 to produce transient target inhibition and potentially improve the therapeutic window. From these efforts, a series of inhibitors were developed including, KPT-330, also known as selinexor. In preclinical studies, selinexor treatment inhibits XPO1 with nuclear accumulation of its targets. In addition, selinexor reduces XPO1 levels through proteasomal degradation [45]. Studies with Selinexor and other SINEs provide further novel insights on XPO1’s effects in tumor cells. Multiple mechanisms, some interconnected, affecting cell cycle regulation, apoptosis, stress response, and immune evasion are observed. A study in myeloma cells with KPT-185 or KPT-330 shows changes in cell cycle regulators and apoptosis, nuclear accumulation of p53, p27, p21 and FOXO3A, nuclear accumulation of IkB, affecting NF-kB signaling, reduction in c-MYC and antiapoptotic proteins Mcl-1 and Bcl-xL and increase in mRNA of stress response proteins [11, 13]. Interestingly, XPO1 inhibition might also affect the tumor microenvironment with antitumor effects [13].
Consistent with these biological effects, selinexor reduces the growth and viability of many solid tumors, non-Hodgkin lymphoma, myeloma and leukemia cells in vitro and in vivo [16, 30, 46, 47]. Notably, studies in AML and ALL have suggest selinexor also targets the leukemic stem cells, a small subpopulation of leukemia cells, which are thought drive the relapse in AML [48, 49]. Selinexor treatment reduces engraftment in a mouse leukemia xenograft model and reduces frequencies of LSC in selinexor-treated xenografts [50].
Next generation XPO1 inhibitor, KPT-8602, was developed to reduce central nervous system- related toxicity of selinexor, by reducing its blood–brain barrier penetration. KPT-8602 shows similar efficacy to selinexor against AML and ALL cells in vitro and in mouse xenograft models. Initial clinical trials show high antitumor activity and better tolerability [51, 52].
Preclinical trials combining SINEs with conventional treatments
The understanding of XPO1 regulation of specific cargoes provides the rationale for testing the combination of SINEs with conventional treatments with expected synergism or as a way to overcome resistance.
In vitro studies of XPO1 inhibition in combination with current myeloma treatments find that targeting XPO1 overcomes resistance to proteasome inhibitors. Proteasome inhibitors such as bortezomib and carfilzomib target NF-kB by inhibiting IkB-α proteasomal degradation. Inhibition of XPO1 results in nuclear retention of IkB-α, which might protect it from proteasomal degradation in the cytoplasm. Further studies, performed in myeloma cells from proteasome inhibitor-refractory patients, show that treatment with selinexor results in nuclear retention of IkB-α, along with increase in its total levels. In accord, XPO1 inhibition results in a restored sensitivity to proteasome inhibitors and detection of NF-kB- IkB-α complexes [53]. The combination of selinexor with dexamethasone, which achieved FDA approval, is also based on preclinical studies showing that XPO1 inhibition induces nuclear retention of phosphorylated glucocorticoid receptor, augmenting its activity. Combing dexamethasone with selinexor shows a synergistic effect with inhibition of the mTOR pathway and augmented myeloma cell death [54].
Several preclinical studies have investigated how XPO1 inhibition potentiate common treatment in AML. As mentioned, Topo IIa is one of the cargoes of XPO1, and cytoplasmic localization of Topo II leads to resistance to common Topo inhibitors such as anthracyclines. Treatment with selinexor restores Topo II nuclear localization in AML cell lines and primary AML cells and sensitizes them to treatment with anthracyclines [55]. Another study combines the DNA methyltransferase decitabine with selinexor. While no synergistic effect was noted by combining the two drugs, pretreatment with decitabine sensitizes leukemia cells to selinexor. This effect is presumably by upregulation of key tumor-suppressor genes by decitabine such as CDKN1A and FOXO3A, which are targets of XPO1 [56].
Venetoclax, a selective inhibitor of Bcl-2, is incorporated in the treatment of AML, among other hematological malignancies. Venetoclax treatment reduces the interaction of the proapoptotic protein Bim with BCL-2, however the interaction of Bim with the antiapoptotic protein Mcl-1 is thought to mediate resistance to venetoclax [57]. Treatment with selinexor reduces the levels of Mcl-1, and shows a synergistic effect when combined with venetoclax in AML cells [58].
Targeting XPO1 in AML also shows activity against FLT3-mutated AML cells. FLT3-mutation results in a constitutively active FLT3 signaling, and carries an adverse prognosis with highly proliferative disease. Interestingly, cells with mutated FLT3, regardless of the type of mutation, are more sensitive to XPO1 inhibition, as compared to cells with wild-type FLT3. Further studies into the activity of FLT3 following XPO1 inhibition reveal a surprising compensatory upregulation of phosphorylated FLT3 and its downstream signaling targets. To overcome this, selinexor is combined with FLT3 inhibitor sorafenib, resulting in a marked synergistic effect in vitro and in vivo [59].
In CML, the fusion BCR-ABL protein deregulated kinase activity is mainly limited to the cytoplasm, while treatment with imatinib results in its nuclear localization [60]. Leptomycin B inhibits BCR-ABL export by XPO1 and sensitizes CML cells to imatinib, resulting in reduction in growth and viability [61]. Furthermore, inhibition of XPO1 preferentially targets CD34+ cells form CML patients compared to cord blood control, including CD34+ leukemic blasts from blast crisis CML, which suggests another therapeutic option in this challenging patient population [62].
Treatment of CLL cells with selinexor reduces proliferation of leukemic cells and abrogates phosphorylation of AKT and ERK, reflecting inhibition of BTK activation [63]. Ibrutinib is a BTK inhibitor in clinical use in CLL and other B cell malignancies. Relapse after ibrutinib is often due to the C481S BTK mutation that makes its binding to BTK reversible. Selinexor is also effective in primary CLL cells from patients that relapse after ibrutinib with the C481S mutation [64]. The latter activity of selinexor is also observed in ibrutinib-resistant MCL lines [36].
Clinical trials with nuclear transport inhibitors
Lessons from preclinical studies with SINEs as single agent and in combinations mentioned above guided the development of clinical studies (Table 2).
Multiple myeloma
Selinexor is evaluated in a heavily pretreated MM patients either alone or in combination with dexamethasone. The study includes also a small number of patients with Waldenstrom Macroglobulinemia. Of the 84 patients, overall response rate is 10% with a median duration of response of 5 months. The combination of dexamethasone with selinexor shows a better overall response rate compared with selinexor alone (22% vs 4%) [65]. Selinexor–dexamethasone is further evaluated in triple-class refractory patients shows a partial response in 26% of the patients, with two complete response. Median duration of response is 4.4 months [66]. Similar results are reported from the phase II STORM trial, looking at an even more refractory patient population which progressed on bortezomib, carfilzomib, lenalidomide, and pomalidomide, also reports similar ORR rates (20%), including penta-refractory patients, and similar duration of response (5 months) [67]. The later trial has led to FDA approval of selinexor in combination with dexamethasone for relapsed-refractory multiple myeloma. The selinexor and backbone treatments of multiple myeloma trial is a 9-arm study, evaluating selinexor–dexamethasone in combination with various treatments, including pomalidomide, bortezomib, lenalidomide, ixazomib, and elotuzumab in different combinations (NCT02343042). The results will undoubtfully improve our understanding on how to incorporate selinexor in terms of efficacy and toxicity. The combination of selinexor with low-dose bortezomib (1.3 mg/m2) and dexamethasone (SVd) in relapsed refractory MM patients is published, with overall response rate of 63% and 43% for proteasome inhibitor-refractory patients, and PFS of 9 and 6 months, respectively [68]. A different study evaluates selinexor–carfilzomib and dexamethasone, with ORR of 48% in heavily pretreated patients. Interestingly, a very good partial response of 15% is reported for patients who are refractory to carfilzomib in the last line of treatment [69]. Selinexor is also being studied in combination with melphalan before stem cell transplant (NCT02780609).
Common side effects, observed in all clinical trials include hematological toxicity, constitutional and gastrointestinal adverse effects and hyponatremia. Thrombocytopenia is the most common cytopenia, it is dose dependent and occurs in up to 60% of the patients, with 15% grade 3/4 [70]. Interestingly, the mechanism of thrombocytopenia involves the inhibition of megakaryocyte maturation, which might again reflects its ability to target progenitor cells [71]. Constitutional symptoms include nausea, fatigue, anorexia and weight loss and are reported in 40–70% of the patients with most being grades 1–2, as in the STORM study [67]. Grade 3 hyponatremia is reported in 22% of the patients, but is generally asymptomatic and reversible with supportive care. Taken together, dose interruptions for adverse effects are reported in 52% in the STORM study [67]
Acute leukemia
A phase 1 trial in 95 patients with relapsed refractory AML reports over 50% reduction in bone marrow blasts in 30% of the evaluated patients. The response also translates to an improved PFS (5.1 vs 1.3 months) and OS improvement (9.7 vs. 2.7 months). Common toxicities include fatigue and gastrointestinal, which are primarily grade 1 and 2. The most frequent grades 3 and 4 adverse events are hematological and fatigue. No deaths are reported directly from treatment [72]. Preclinical data highlight several mechanisms of synergism with conventional drugs in AML as well as the ability to overcome resistance. The combination of selinexor with decitabine in relapsed refractory AML patients has an overall response rate of 40%. Adverse events include again hematological toxicities, however a high rate of hyponatremia has been reported [73]. Two trial report the combination of selinexor with high-dose chemotherapy. The combination of high-dose cytarabine and mitoxantrone in 12 newly diagnosed patients and 8 relapsed patients has an ORR rate of 92% and 38%, respectively. Response rate is higher for 80 mg of selinexor compared with 60 mg. Again, myelosuppression is the most common adverse event, with longer than expected count recovery time, resulting in more hemorrhagic and infectious events [74]. Combination of selinexor with 7 + 3 protocol in 21 newly diagnosed poor risk AML patients results in an ORR of 53% with 42% CR rate. Toxicity profile is similar [75]. Further phase 3 studies are necessary to accurately determine the role of selinexor in AML treatment scheme, especially in the newly diagnosed setting. The combination of selinexor with venetoclax is currently evaluated in an on-going study in relapsed refractory patients with AML and non-Hodgkin lymphoma (NCT02485535).
Non-Hodgkin lymphoma
A phase I study included 79 patients, 54 patients with DLBCL and the rest with other NHL types including: follicular lymphoma, CLL, MCL, cutaneous T cell lymphoma and Burkitt’s lymphoma. Most patients have two or more prior treatments. ORR is ~30% and 4 patients with DLBCL achieve CR, out of 41 evaluated patients. Response rates are similar in germinal center B-cell like (GCB) and non-GCB subtypes. Of six patients with double hit or triple hit lymphoma, 3 respond, 1 with CR. For the patients that achieve CR, response appears durable, with up to 35 months at the time of the report [47]. Selinexor has excellent CNS penetration, and has been evaluated in patients with glioblastoma. A recent case report describes a case of DLBCL with isolated relapse in the CNS. The patient received several lines of treatment including autologous stem cell transplantation prior to the relapse, and failed to respond to methotrexate. Treatment with selinexor results in complete remission within 5 months of treatment [76].
Key pathways regulated by nuclear import and their significance in hematological malignancies
Overexpression of both IPOα and IPOβ has been reported in non-Hodgkin lymphoma [77], myeloma [78], and leukemia [79] as well as many solid tumors, and is frequently correlated with poor prognosis and a more aggressive tumor phenotype [1, 80]. Further studies have highlighted how nuclear transport affect the function of key oncogenes and tumor-suppressor genes, providing a better understanding of tumor biology and novel targets for therapeutic designs.
One of the best studied examples of how IPOs promote tumorigenesis is the NF-kB pathway. NF-kB are a family of transcription factors whose transcriptional targets promote tumor cells proliferation, inhibit apoptosis and stimulate angiogenesis and tumor invasiveness. Not surprisingly, a high NF-kB activity is observed in solid tumors [81] and hematological malignancies including multiple myeloma, DLBCL, AML, CML, and MDS [82]. The p50/p65 heterodimer is considered the canonical NF-kB transcription factor and its nuclear localization is mediated by IPOβ1. In myeloma cells, genetically silencing IPOβ1 or its inhibition with the IPOβ1 inhibitor importazole (IPZ) reduces the nuclear localization of p65. In accord, the expression of NF-kB signaling target genes are reduced with resulting reduction in growth and viability [78]. IPOβ1 expression in DLBCL is also significantly higher compared with normal B cells, and is associated with higher proliferation, NF-kB activation and shorter overall survival [83].
The Janus Kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is often activated in both myeloid and lymphoid malignancies. Signal transduction is dependent on nuclear import of several of its key players and is dependent on IPOα [84]. JAK1 is especially interesting due its significance in ABC (activated B cell like) DLBCL cells. Silencing of JAK1 reduces viability of DLBCL cells and this effect cannot be rescued by a NLS-mutated JAK1, demonstrating the significance of its nuclear localization [85]. ABC DLBCL has a high relapse rate with adverse prognosis. Thus, dual targeting of the JAK/STAT pathway at the kinase level and by blocking its nuclear import might prove a highly effective therapeutic option. Another example linking nuclear import with key cancer pathways is the wnt pathway. Wnt signal transduction culminates in the IPOβ1-mediated nuclear import of β-catenin [86]. Deregulated wnt signaling has been reported in various lymphoma subtypes, myeloma, and leukemia [87], and depletion of IPOβ1 resulted in G1 arrest and concomitant decrease in β-catenin expression [88]. Finally, IPO7 is linked to B cell receptor (BCR) activation through nuclear shuttling of SYK, which might provide another way to target BCR activation and SYK in hematological malignancies [89].
In leukemia, several studies have demonstrated the significance of nuclear import in driving leukemic pathways. PML/RARa, the hallmark fusion protein of acute promyelocytic leukemia, undergoes cleavage to generate an NLS bearing truncated RARα (NLS-RARα). NLS-RARα is recognized and transported to the nucleus, by the IPOα/IPOβ complex [90], where it promotes proliferation and inhibit differentiation of leukemia cells [91]. Similarly, in t(8;21- AML-1/ETO) AML, ETO bears a NLS sequence that is recognized by IPOα/β complex and mediates ETO and AML-1/ETO import into the nucleus [92]. High levels of IPOβ1 and its adapter IPOα1 are also detected in bone marrow mononuclear cells derived from CML patients. Silencing of IPOβ1 reduces proliferation and induces apoptosis in CML cells, possibly by decreasing the nuclear localization of the transcription factor E2F1 [93].
Other than the IPOα/β complex, certain IPOs are highlighted in acute leukemia due to their specific cargoes or expression pattern. IPO8 mediates the nuclear localization of Eukaryotic translation initiation factor (eIF4E). eIF4E mediates the nuclear export and cytoplasmic translation of certain mRNAs, many of which are known oncogenes [94]. Silencing of IPO8 results in nuclear accumulation of eIF4E mRNA export targets, such as c-Myc and Mcl-1, with a concomitant reduction in their protein levels. Interestingly, IPO8 is an eIF4E mRNA export target, which further supports the increase of IPO8 levels [95].
Finally, a few sporadic reports link IPOs to acute lymphoblastic leukemia (ALL). A nonsense mutation in MED12, which is part of the transcription-regulation mediator complex, is identified in ALL. The mutation disrupts the NLS sequence of MED12, preventing its recognition by IPOα and its nuclear localization and function [96]. In another study, genetic analysis of ALL blasts prior to treatment found that the IPOβ1 gene contributes to the development of secondary brain tumors [97]. The significance of these reports awaits further studies.
Development of nuclear import inhibitors
The wide overexpression and significance of IPOα1 and IPOβ1 in numerous malignancies suggests that cancer cells require a higher volume of IPOα/β-mediated traffic across the nuclear membrane. This might reflect a higher proliferative or metabolic state of the cancer cell, or as mentioned above, the transport of cargoes directly involved in the tumor pathogenesis [1, 80]. Given the importance of the nuclear import pathway and the clinical success of inhibiting nuclear export at the level of XPO1, there has been interest in developing nuclear import inhibitors.
As IPOβ1 is a primary regulator of nuclear import and impacts the import of a wide and diverse range of cargo, initial attention focused on developing IPOβ1 inhibitor. Early efforts to develop an IPOβ1 inhibitor sought to target the binding of RanGTP to IPOβ1. A screen for compounds that interfere with the interaction between RanGTP and IPOβ1 identified importazloe. Importazole is a 2,4-diaminoquinazoline that shows high specificity against IPOβ1 and good cell permeability [98]. Importazole has in vitro efficacy in solid tumors and several hematological malignancies including CML and MM [78, 93, 99]. INI-43 (3-(1H-benzimidazol-2-yl)-1-(3-dimethylaminopropyl) pyrrolo[5,4-b]quinoxalin-2-amine) was identified by a structure based in silico screen to identify compounds that bind the overlapping Ran- and IPOα-binding region of IPOβ1. INI-43 inhibits IPOβ1 with a resulting reduction in nuclear localization of p65, an IPOβ1 cargo. Preliminary proof of concept experiments shows that treatment of several solid cancer cell lines, results in cell cycle arrest and cell death. Furthermore, INI-43 treatment inhibits tumor growth in cancer xenograft models, providing a clear evidence of the potential inhibiting nuclear import in cancer [100].
While the transition into clinical studies with import inhibitors has currently not been successful, they hold great promise as anticancer therapeutic approach. One crucial lesson from the development of XPO1 inhibitors is that while irreversible inhibition by leptomycin B produced severe toxicity, development of inhibitors that allow physiological export had much less toxicity, but maintain efficacy in tumor cells. Thus, development of inhibitors that partially inhibit or slow nuclear import might still have effect in a cancer cells, while less toxic to normal tissues. Stepping away from broad inhibition of nuclear import, our growing understanding of how specific IPO and cargo support certain tumors, might allow to develop cargo-specific inhibitors which rely on its unique NLS. Generating small inert peptides with the same NLS competing for IPO binding site, might have a highly specific activity in tumor cells with less toxicity. Another approach might target the interaction of IPO with the NPC. The specificity of IPO–NPC interaction with regard to tumor and cargo is a growing area of study, which might provide the ability selectively inhibit certain cargoes with limited collateral toxicity.
Conclusion remarks
Transport across the nuclear membrane is at the crossroads of numerous and diverse cellular function, including key elements of cancer development. The proteins that mediate this process, the IPOs, are not merely bystanders but active participants and modulate these processes. Their role in tumorigenesis is evident not only by their wide dysregulation in many hematological malignancies, but also in view of their involvement with key signal transduction pathways, oncogenes, and tumor-suppressor genes. Moreover, data suggest that dysregulation of IPOs and exportins often occur at early stages of tumor development. While there are ample of data on exportins, the role of IPOs is gaining significance as more studies prove their significance. This is especially relevant in treatment combination that target cancer proteins both in their direct function and their subcellular localization. This approach generates a synergistic effect and can also overcome resistance to current treatments. To date, inhibitors of nuclear export are approved for clinical use, but generating potent and specific IPO inhibitors has proven more challenging. Nonetheless, targeting this pathway, both in nuclear import and export still bears great promise as an intervention point with novel therapeutic modalities and merits further study.
References
Çağatay T, Chook YM. Karyopherins in cancer. Curr Opin Cell Biol. 2018;52:30–42.
Stawicki S, Steffen J. The nuclear pore complex: a comprehensive review of structure and function. Int J Acad Med. 2017;3:24.
Mackmull M-T, Klaus B, Heinze I, Chokkalingam M, Beyer A, Russell RB, et al. Landscape of nuclear transport receptor cargo specificity. Mol Syst Biol. 2017;13:962.
Oka M, Yoneda Y. Importin α: functions as a nuclear transport factor and beyond. Proc Jpn Acad Ser B Phys Biol Sci. 2018;94:259–74.
Kim YH, Han M-E, Oh S-O. The molecular mechanism for nuclear transport and its application. Anat Cell Biol. 2017;50:77–85.
Hodel MR, Corbett AH, Hodel AE. Dissection of a nuclear localization signal. J Biol Chem. 2001;276:1317–25.
Lee BJ, Cansizoglu AE, Süel KE, Louis TH, Zhang Z, Chook YM. Rules for nuclear localization sequence recognition by karyopherin beta 2. Cell. 2006;126:543–58.
Fung HYJ, Chook YM. Atomic basis of CRM1-cargo recognition, release and inhibition. Semin Cancer Biol. 2014;27:52–61.
Güttler T, Görlich D. Ran-dependent nuclear export mediators: a structural perspective. EMBO J. 2011;30:3457–74.
Sun Q, Chen X, Zhou Q, Burstein E, Yang S, Jia D. Inhibiting cancer cell hallmark features through nuclear export inhibition. Signal Transduct Target Ther. 2016;1:16010.
Schmidt J, Braggio E, Kortuem KM, Egan JB, Zhu YX, Xin CS, et al. Genome-wide studies in multiple myeloma identify XPO1/CRM1 as a critical target validated using the selective nuclear export inhibitor KPT-276. Leukemia. 2013;27:2357–65.
Wang AY, Liu H. The past, present, and future of CRM1/XPO1 inhibitors. Stem Cell Investig. 2019;6:6.
Tai Y-T, Landesman Y, Acharya C, Calle Y, Zhong MY, Cea M, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014;28:155–65.
Kojima K, Kornblau SM, Ruvolo V, Dilip A, Duvvuri S, Davis RE, et al. Prognostic impact and targeting of CRM1 in acute myeloid leukemia. Blood. 2013;121:4166–74.
Luo B, Huang L, Gu Y, Li C, Lu H, Chen G, et al. Expression of exportin-1 in diffuse large B-cell lymphoma: immunohistochemistry and TCGA analyses. Int J Clin Exp Pathol. 2018;11:5547–60.
Lapalombella R, Sun Q, Williams K, Tangeman L, Jha S, Zhong Y, et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood. 2012;120:4621–34.
Zhang K, Wang M, Tamayo AT, Shacham S, Kauffman M, Lee J, et al. Novel selective inhibitors of nuclear export CRM1 antagonists for therapy in mantle cell lymphoma. Exp Hematol. 2013;41:67–78.e4.
Yoshimura M, Ishizawa J, Ruvolo V, Dilip A, Quintás-Cardama A, McDonnell TJ, et al. Induction of p53-mediated transcription and apoptosis by exportin-1 (XPO1) inhibition in mantle cell lymphoma. Cancer Sci. 2014;105:795–801.
Walker CJ, Oaks JJ, Santhanam R, Neviani P, Harb JG, Ferenchak G, et al. Preclinical and clinical efficacy of XPO1/CRM1 inhibition by the karyopherin inhibitor KPT-330 in Ph+ leukemias. Blood. 2013;122:3034–44.
Golomb L, Bublik DR, Wilder S, Nevo R, Kiss V, Grabusic K, et al. Importin 7 and exportin 1 link c-Myc and p53 to regulation of ribosomal biogenesis. Mol Cell. 2012;45:222–32.
Bond J, Bergon A, Durand A, Tigaud I, Thomas X, Asnafi V, et al. Cryptic XPO1-MLLT10 translocation is associated with HOXA locus deregulation in T-ALL. Blood. 2014;124:3023–5.
Jardin F, Pujals A, Pelletier L, Bohers E, Camus V, Mareschal S, et al. Recurrent mutations of the exportin 1 gene (XPO1) and their impact on selective inhibitor of nuclear export compounds sensitivity in primary mediastinal B-cell lymphoma: XPO1 Mutations in Primary Mediastinal B-Cell Lymphoma. Am J Hematol. 2016;91:923–30.
Camus V, Miloudi H, Taly A, Sola B, Jardin F. XPO1 in B cell hematological malignancies: from recurrent somatic mutations to targeted therapy. J Hematol Oncol. 2017;10:47.
Camus V, Stamatoullas A, Mareschal S, Viailly P-J, Sarafan-Vasseur N, Bohers E, et al. Detection and prognostic value of recurrent exportin 1 mutations in tumor and cell-free circulating DNA of patients with classical Hodgkin lymphoma. Haematologica. 2016;101:1094–101.
Jeromin S, Weissmann S, Haferlach C, Dicker F, Bayer K, Grossmann V, et al. SF3B1 mutations correlated to cytogenetics and mutations in NOTCH1, FBXW7, MYD88, XPO1 and TP53 in 1160 untreated CLL patients. Leukemia. 2014;28:108–17.
Senapedis WT, Baloglu E, Landesman Y. Clinical translation of nuclear export inhibitors in cancer. Semin Cancer Biol. 2014;27:74–86.
Liang S-H, Clarke MF. Regulation of p53 localization: subcellular localization of p53. Eur J Biochem. 2001;268:2779–83.
Lu W, Pochampally R, Chen L, Traidej M, Wang Y, Chen J. Nuclear exclusion of p53 in a subset of tumors requires MDM2 function. Oncogene. 2000;19:232–40.
Han X, Wang J, Shen Y, Zhang N, Wang S, Yao J, et al. CRM1 as a new therapeutic target for non-Hodgkin lymphoma. Leuk Res. 2015;39:38–46.
Gandhi UH, Senapedis W, Baloglu E, Unger TJ, Chari A, Vogl D, et al. Clinical implications of targeting XPO1-mediated nuclear export in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2018;18:335–45.
Kashyap T, Argueta C, Aboukameel A, Unger TJ, Klebanov B, Mohammad RM, et al. Selinexor, a selective inhibitor of nuclear export (SINE) compound, acts through NF-κB deactivation and combines with proteasome inhibitors to synergistically induce tumor cell death. Oncotarget. 2016;7:78883–95.
Turner JG, Dawson J, Emmons MF, Cubitt CL, Kauffman M, Shacham S, et al. CRM1 inhibition sensitizes drug resistant human myeloma cells to topoisomerase II and proteasome inhibitors both in vitro and ex vivo. J Cancer. 2013;4:614–25.
Falini B, Bolli N, Shan J, Martelli MP, Liso A, Pucciarini A, et al. Both carboxy-terminus NES motif and mutated tryptophan(s) are crucial for aberrant nuclear export of nucleophosmin leukemic mutants in NPMc+ AML. Blood. 2006;107:4514–23.
Brunetti L, Gundry MC, Sorcini D, Guzman AG, Huang Y-H, Ramabadran R, et al. Mutant NPM1 maintains the leukemic state through HOX expression. Cancer Cell. 2018;34:499–512.e9.
Ranganathan P, Yu X, Na C, Santhanam R, Shacham S, Kauffman M, et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood. 2012;120:1765–73.
Ming M, Wu W, Xie B, Sukhanova M, Wang W, Kadri S, et al. XPO1 inhibitor selinexor overcomes intrinsic ibrutinib resistance in mantle cell lymphoma via nuclear retention of IκB. Mol Cancer Ther. 2018;17:2564–74.
Tabe Y, Kojima K, Yamamoto S, Sekihara K, Matsushita H, Davis RE, et al. Ribosomal biogenesis and translational flux inhibition by the selective inhibitor of nuclear export (SINE) XPO1 antagonist KPT-185. PLoS ONE. 2015;10:e0137210.
Miloudi H, Leroy K, Jardin F, Sola B. STAT6 is a cargo of exportin 1: Biological relevance in primary mediastinal B-cell lymphoma. Cell Signal. 2018;46:76–82.
Kutay U, Bischoff FR, Kostka S, Kraft R, Görlich D. Export of importin alpha from the nucleus is mediated by a specific nuclear transport factor. Cell. 1997;90:1061–71.
Jiang M-C. CAS (CSE1L) signaling pathway in tumor progression and its potential as a biomarker and target for targeted therapy. Tumour Biol J Int Soc Oncodev Biol Med. 2016;37:13077–90.
Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA. 1999;96:9112–7.
Sun Q, Carrasco YP, Hu Y, Guo X, Mirzaei H, Macmillan J, et al. Nuclear export inhibition through covalent conjugation and hydrolysis of Leptomycin B by CRM1. Proc Natl Acad Sci USA. 2013;110:1303–8.
Newlands ES, Rustin GJ, Brampton MH. Phase I trial of elactocin. Br J Cancer. 1996;74:648–9.
Dickmanns A, Monecke T, Ficner R. Structural basis of targeting the exportin CRM1 in. Cancer Cells. 2015;4:538–68.
Mendonca J, Sharma A, Kim H-S, Hammers H, Meeker A, De Marzo A, et al. Selective inhibitors of nuclear export (SINE) as novel therapeutics for prostate cancer. Oncotarget. 2014;5:6102–12.
Abeykoon JP, Paludo J, Nowakowski KE, Stenson MJ, King RL, Wellik LE, et al. The effect of CRM1 inhibition on human non-Hodgkin lymphoma cells. Blood Cancer J. 2019;9:24.
Kuruvilla J, Savona M, Baz R, Mau-Sorensen PM, Gabrail N, Garzon R, et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma. Blood. 2017;129:3175–83.
Shlush LI, Mitchell A, Heisler L, Abelson S, Ng SWK, Trotman-Grant A, et al. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature. 2017;547:104–8.
Chopra M, Bohlander SK. The cell of origin and the leukemia stem cell in acute myeloid leukemia. Genes Chromosom Cancer. 2019;58:850–8.
Etchin J, Montero J, Berezovskaya A, Le BT, Kentsis A, Christie AL, et al. Activity of a selective inhibitor of nuclear export, selinexor (KPT-330), against AML-initiating cells engrafted into immunosuppressed NSG mice. Leukemia. 2016;30:190–9.
Vercruysse T, De Bie J, Neggers JE, Jacquemyn M, Vanstreels E, Schmid-Burgk JL, et al. The second-generation exportin-1 inhibitor KPT-8602 demonstrates potent activity against acute lymphoblastic leukemia. Clin Cancer Res J Am Assoc Cancer Res. 2017;23:2528–41.
Etchin J, Berezovskaya A, Conway AS, Galinsky IA, Stone RM, Baloglu E, et al. KPT-8602, a second-generation inhibitor of XPO1-mediated nuclear export, is well tolerated and highly active against AML blasts and leukemia-initiating cells. Leukemia. 2017;31:143–50.
Turner JG, Kashyap T, Dawson JL, Gomez J, Bauer AA, Grant S, et al. XPO1 inhibitor combination therapy with bortezomib or carfilzomib induces nuclear localization of IκBα and overcomes acquired proteasome inhibitor resistance in human multiple myeloma. Oncotarget. 2016;7:78896–909.
Argueta C, Kashyap T, Klebanov B, Unger TJ, Guo C, Harrington S, et al. Selinexor synergizes with dexamethasone to repress mTORC1 signaling and induce multiple myeloma cell death. Oncotarget. 2018;9:25529–44.
Ranganathan P, Kashyap T, Yu X, Meng X, Lai T-H, McNeil B, et al. XPO1 inhibition using selinexor synergizes with chemotherapy in acute myeloid leukemia by targeting DNA repair and restoring topoisomerase IIα to the nucleus. Clin Cancer Res J Am Assoc Cancer Res. 2016;22:6142–52.
Ranganathan P, Yu X, Santhanam R, Hofstetter J, Walker A, Walsh K, et al. Decitabine priming enhances the antileukemic effects of exportin 1 (XPO1) selective inhibitor selinexor in acute myeloid leukemia. Blood. 2015;125:2689–92.
Niu X, Zhao J, Ma J, Xie C, Edwards H, Wang G, et al. Binding of released bim to Mcl-1 is a mechanism of intrinsic resistance to ABT-199 which can be overcome by combination with daunorubicin or cytarabine in AML cells. Clin Cancer Res J Am Assoc Cancer Res. 2016;22:4440–51.
Luedtke DA, Su Y, Liu S, Edwards H, Wang Y, Lin H, et al. Inhibition of XPO1 enhances cell death induced by ABT-199 in acute myeloid leukaemia via Mcl-1. J Cell Mol Med. 2018;22:6099–111.
Zhang W, Ly C, Ishizawa J, Mu H, Ruvolo V, Shacham S, et al. Combinatorial targeting of XPO1 and FLT3 exerts synergistic anti-leukemia effects through induction of differentiation and apoptosis in FLT3-mutated acute myeloid leukemias: from concept to clinical trial. Haematologica. 2018;103:1642–53.
Ren R. Mechanisms of BCR–ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5:172–83.
Vigneri P, Wang JYJ. Induction of apoptosis in chronic myelogenous leukemia cells through nuclear entrapment of BCR–ABL tyrosine kinase. Nat Med. 2001;7:228–34.
Khorashad JS, Eiring AM, Mason CC, Gantz KC, Bowler AD, Redwine HM, et al. shRNA library screening identifies nucleocytoplasmic transport as a mediator of BCR-ABL1 kinase-independent resistance. Blood. 2015;125:1772–81.
Zhong Y, El-Gamal D, Dubovsky JA, Beckwith KA, Harrington BK, Williams KE, et al. Selinexor suppresses downstream effectors of B-cell activation, proliferation and migration in chronic lymphocytic leukemia cells. Leukemia. 2014;28:1158–63.
Hing ZA, Mantel R, Beckwith KA, Guinn D, Williams E, Smith LL, et al. Selinexor is effective in acquired resistance to ibrutinib and synergizes with ibrutinib in chronic lymphocytic leukemia. Blood. 2015;125:3128–32.
Chen C, Siegel D, Gutierrez M, Jacoby M, Hofmeister CC, Gabrail N, et al. Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and Waldenstrom macroglobulinemia. Blood. 2018;131:855–63.
Chari A, Vogl DT, Gavriatopoulou M, Nooka AK, Yee AJ, Huff CA, et al. Oral Selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381:727–38.
Vogl DT, Dingli D, Cornell RF, Huff CA, Jagannath S, Bhutani D, et al. Selective inhibition of nuclear export with oral selinexor for treatment of relapsed or refractory multiple myeloma. J Clin Oncol J Am Soc Clin Oncol. 2018;36:859–66.
Bahlis NJ, Sutherland H, White D, Sebag M, Lentzsch S, Kotb R, et al. Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood. 2018;132:2546–54.
Jakubowiak AJ, Jasielec JK, Rosenbaum CA, Cole CE, Chari A, Mikhael J, et al. Phase 1 study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Br J Haematol. 2019;186:549–60.
Abdul Razak AR, Mau-Soerensen M, Gabrail NY, Gerecitano JF, Shields AF, Unger TJ, et al. First-in-class, first-in-human phase I study of selinexor, a selective inhibitor of nuclear export, in patients with advanced solid tumors. J Clin Oncol J Am Soc Clin Oncol. 2016;34:4142–50.
Machlus KR, Wu SK, Vijey P, Soussou TS, Liu Z-J, Shacham E, et al. Selinexor-induced thrombocytopenia results from inhibition of thrombopoietin signaling in early megakaryopoiesis. Blood. 2017;130:1132–43.
Garzon R, Savona M, Baz R, Andreeff M, Gabrail N, Gutierrez M, et al. A phase 1 clinical trial of single-agent selinexor in acute myeloid leukemia. Blood. 2017;129:3165–74.
Bhatnagar B, Zhao Q, Mims AS, Vasu S, Behbehani GK, Larkin K, et al. Selinexor in combination with decitabine in patients with acute myeloid leukemia: results from a phase 1 study. Leuk Lymphoma. 2020;61:387–96.
Wang AY, Weiner H, Green M, Chang H, Fulton N, Larson RA, et al. A phase I study of selinexor in combination with high-dose cytarabine and mitoxantrone for remission induction in patients with acute myeloid leukemia. J Hematol OncolJ Hematol Oncol. 2018;11:4.
Sweet K, Komrokji R, Padron E, Cubitt CL, Turner JG, Zhou J, et al. Phase I clinical trial of selinexor in combination with daunorubicin and cytarabine in previously untreated poor-risk acute myeloid leukemia. Clin Cancer Res. 2020;26:54–60.
Bobillo S, Abrisqueta P, Carpio C, Raheja P, Castellví J, Crespo M, et al. Promising activity of selinexor in the treatment of a patient with refractory diffuse large B-cell lymphoma and central nervous system involvement. Haematologica. 2018;103:e92–e93.
He S, Miao X, Wu Y, Zhu X, Miao X, Yin H, et al. Upregulation of nuclear transporter, Kpnβ1, contributes to accelerated cell proliferation- and cell adhesion-mediated drug resistance (CAM-DR) in diffuse large B-cell lymphoma. J Cancer Res Clin Oncol. 2016;142:561–72.
Yan W, Li R, He J, Du J, Hou J. Importin β1 mediates nuclear factor-κB signal transduction into the nuclei of myeloma cells and affects their proliferation and apoptosis. Cell Signal. 2015;27:851–9.
Nachmias B, Voisin V, Hurren R, Wang X, Maclean N, Xu GW, et al. IPO11 is upregulated in relapsed AML and supports survival of leukemic stem cells. Blood. 2019;134:2530–2530.
Han Y, Wang X. The emerging roles of KPNA2 in cancer. Life Sci. 2020;241:117140.
Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2:823–30.
Cilloni D, Martinelli G, Messa F, Baccarani M, Saglio G. Nuclear factor B as a target for new drug development in myeloid malignancies. Haematologica. 2007;92:1224–9.
He S, Miao X, Wu Y, Zhu X, Miao X, Yin H, et al. Upregulation of nuclear transporter, Kpnβ1, contributes to accelerated cell proliferation- and cell adhesion-mediated drug resistance (CAM-DR) in diffuse large B-cell lymphoma. J Cancer Res Clin Oncol. 2016;142:561–72.
Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32:2601–13.
Zhu F, Hwang B, Miyamoto S, Rui L. Nuclear import of JAK1 is mediated by a classical NLS and is required for survival of diffuse large B-cell lymphoma. Mol Cancer Res. 2017;15:348–57.
Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012; 4. https://doi.org/10.1101/cshperspect.a008052.
Grainger S, Traver D, Willert K. Wnt signaling in hematological malignancies. Prog Mol Biol Transl Sci. 2018;153:321–41.
Lu T, Bao Z, Wang Y, Yang L, Lu B, Yan K, et al. Karyopherinβ1 regulates proliferation of human glioma cells via Wnt/β-catenin pathway. Biochem Biophys Res Commun. 2016;478:1189–97.
Mohammad DK, Nore BF, Gustafsson MO, Mohamed AJ, Smith CIE. Protein kinase B (AKT) regulates SYK activity and shuttling through 14-3-3 and importin 7. Int J Biochem Cell Biol. 2016;78:63–74.
Ye J, Zhong L, Xiong L, Li J, Yu L, Dan W, et al. Nuclear import of NLS- RARα is mediated by importin α/β. Cell Signal. 2020;69:109567.
Hu X-X, Zhong L, Zhang X, Gao Y-M, Liu B-Z. NLS-RARα promotes proliferation and inhibits differentiation in HL-60 cells. Int J Med Sci. 2014;11:247–54.
Odaka Y, Mally A, Elliott LT, Meyers S. Nuclear import and subnuclear localization of the proto-oncoprotein ETO (MTG8). Oncogene. 2000;19:3584–97.
Wang T, Huang Z, Huang N, Peng Y, Gao M, Wang X, et al. Inhibition of KPNB1 inhibits proliferation and promotes apoptosis of chronic myeloid leukemia cells through regulation of E2F1. OncoTargets Ther. 2019;12:10455–67.
Assouline S, Culjkovic B, Cocolakis E, Rousseau C, Beslu N, Amri A, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114:257–60.
Volpon L, Culjkovic-Kraljacic B, Osborne MJ, Ramteke A, Sun Q, Niesman A, et al. Importin 8 mediates m7G cap-sensitive nuclear import of the eukaryotic translation initiation factor eIF4E. Proc Natl Acad Sci USA. 2016;113:5263–8.
Heikkinen T, Kämpjärvi K, Keskitalo S, von Nandelstadh P, Liu X, Rantanen V, et al. Somatic MED12 nonsense mutation escapes mRNA decay and reveals a motif required for nuclear entry. Hum Mutat. 2017;38:269–74.
Edick MJ, Cheng C, Yang W, Cheok M, Wilkinson MR, Pei D, et al. Lymphoid gene expression as a predictor of risk of secondary brain tumors. Genes Chromosom Cancer. 2005;42:107–16.
Soderholm JF, Bird SL, Kalab P, Sampathkumar Y, Hasegawa K, Uehara-Bingen M, et al. Importazole, a small molecule inhibitor of the transport receptor importin-β. ACS Chem Biol. 2011;6:700–8.
Yang J, Guo Y, Lu C, Zhang R, Wang Y, Luo L, et al. Inhibition of karyopherin beta 1 suppresses prostate cancer growth. Oncogene. 2019;38:4700–14.
van der Watt PJ, Chi A, Stelma T, Stowell C, Strydom E, Carden S, et al. Targeting the nuclear import receptor Kpnβ1 as an anticancer therapeutic. Mol Cancer Ther. 2016;15:560–73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ADS has received honorarium from Novartis, Jazz, and Otsuka Pharmaceuticals and research support from Medivir AB and Takeda. ADS is named as an inventor on a patent application related to the use of DNT cells in AML. ADS owns stock in AbbVie Pharmaceuticals ADS hold the Ronald N. Buick Chair in Oncology Research. BN—no COI to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nachmias, B., Schimmer, A.D. Targeting nuclear import and export in hematological malignancies. Leukemia 34, 2875–2886 (2020). https://doi.org/10.1038/s41375-020-0958-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0958-y
This article is cited by
-
Nuclear transport proteins: structure, function, and disease relevance
Signal Transduction and Targeted Therapy (2023)
-
P53 status, and G2/M cell cycle arrest, are determining factors in cell-death induction mediated by ELF-EMF in glioblastoma
Scientific Reports (2023)
-
In search of new stratification strategies: tissue proteomic profiling of papillary thyroid microcarcinoma in patients with localized disease and lateral neck metastases
Journal of Cancer Research and Clinical Oncology (2023)
-
The importin beta superfamily member RanBP17 exhibits a role in cell proliferation and is associated with improved survival of patients with HPV+ HNSCC
BMC Cancer (2022)
-
Exportin-1 is critical for cell proliferation and survival in adult T cell leukemia
Investigational New Drugs (2022)