Abstract
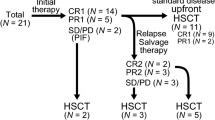
The NOPHO ALL2008 is a population-based study using an unmodified pediatric protocol in patients 1–45 years of age with acute lymphoblastic leukemia. Patients with T-ALL were given a traditional pediatric scheme if fast responding (minimal residual disease (MRD) < 0.1% day 29), or intensive block-based chemotherapy if slow responding (MRD > 0.1% day 29). Both treatment arms included pediatric doses of high-dose methotrexate and asparaginase. If MRD ≥ 5% on day 29 or ≥0.1% after consolidation, patients were assigned to allogeneic hematopoietic stem cell transplantation. The 5-year overall survival of the 278 T-ALL patients was 0.75 (95% CI 0.69–0.81), being 0.82 (0.74–0.88) for patients 1.0–9.9 years, 0.76 (0.66–0.86) for those 10.0–17.9 years, and 0.65 (0.55–0.75) for the older patients. The risk of death in first remission was significantly higher in adults (12%) compared with the 1–9 years group (4%). The MRD responses in the three age groups were similar, and only a nonsignificant increase in relapse risk was found in adults. In conclusion, an unmodified pediatric protocol in patients 1–45 years is effective in all age groups. The traditional pediatric treatment schedule was safe for all patients, but the intensive block therapy led to a high toxic death rate in adults.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Seibel NL. Treatment of acute lymphoblastic leukemia in children and adolescents: peaks and pitfalls. Hematol Am Soc Hematol Educ Program. 2008;2008:374–80.
Pullen DJ, Sullivan MP, Falletta JM, Boyett JM, Humphrey GB, Starling KA, et al. Modified LSA2-L2 treatment in 53 children with E-rosette-positive T-cell leukemia: results and prognostic factors (a Pediatric Oncology Group Study). Blood. 1982;60:1159–68.
Borowitz MJ, Dowell BL, Boyett JM, Pullen DJ, Crist WM, Quddus FM, et al. Clinicopathologic aspects of E rosette negative T cell acute lymphocytic leukemia: a Pediatric Oncology Group study. J Clin Oncol. 1986;4:170–7.
Hastings C, Gaynon PS, Nachman JB, Sather HN, Lu X, Devidas M, et al. Increased post-induction intensification improves outcome in children and adolescents with a markedly elevated white blood cell count (>/=200 × 10(9)/l) with T cell acute lymphoblastic leukaemia but not B cell disease: a report from the Children’s Oncology Group. Br J Haematol. 2015;168:533–46.
Saarinen-Pihkala UM, Gustafsson G, Carlsen N, Flaegstad T, Forestier E, Glomstein A, et al. Outcome of children with high-risk acute lymphoblastic leukemia (HR-ALL): nordic results on an intensive regimen with restricted central nervous system irradiation. Pediatr Blood Cancer. 2004;42:8–23.
Asselin BL, Devidas M, Wang C, Pullen J, Borowitz MJ, Hutchison R, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children’s Oncology Group (POG 9404). Blood. 2011;118:874–83.
Hofmans M, Suciu S, Ferster A, Van Vlierberghe P, Mazingue F, Sirvent N, et al. Results of successive EORTC-CLG 58 881 and 58 951 trials in paediatric T-cell acute lymphoblastic leukaemia (ALL). Br J Haematol. 2019;186:741–53.
Winter SS, Dunsmore KP, Devidas M, Wood BL, Esiashvili N, Chen Z, et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemia: results from the Children’s Oncology Group AALL0434 methotrexate randomization. J Clin Oncol. 2018;36:2926–34.
Marks DI, Paietta EM, Moorman AV, Richards SM, Buck G, DeWald G, et al. T-cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993). Blood. 2009;114:5136–45.
Kantarjian H, Thomas D, O’Brien S, Cortes J, Giles F, Jeha S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–801.
Gokbuget N, Hoelzer D, Arnold R, Bohme A, Bartram CR, Freund M, et al. Treatment of Adult ALL according to protocols of the German Multicenter Study Group for Adult ALL (GMALL). Hematol Oncol Clin North Am. 2000;14:1307–25. ix
Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22:4075–86.
Marks DI, Rowntree C. Management of adults with T-cell lymphoblastic leukemia. Blood. 2017;129:1134–42.
Annino L, Vegna ML, Camera A, Specchia G, Visani G, Fioritoni G, et al. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002;99:863–71.
Toft N, Birgens H, Abrahamsson J, Griskevicius L, Hallbook H, Heyman M, et al. Results of NOPHO ALL2008 treatment for patients 1–45 years with acute lymphoblastic leukemia. Leukemia. 2018;32:606–15.
Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27:911–8.
Hough R, Rowntree C, Goulden N, Mitchell C, Moorman A, Wade R, et al. Efficacy and toxicity of a paediatric protocol in teenagers and young adults with Philadelphia chromosome negative acute lymphoblastic leukaemia: results from UKALL 2003. Br J Haematol. 2016;172:439–51.
DeAngelo DJ, Stevenson KE, Dahlberg SE, Silverman LB, Couban S, Supko JG, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29:526–34.
Storring JM, Minden MD, Kao S, Gupta V, Schuh AC, Schimmer AD, et al. Treatment of adults with BCR-ABL negative acute lymphoblastic leukaemia with a modified paediatric regimen. Br J Haematol. 2009;146:76–85.
Stock W, Luger SM, Advani AS, Yin J, Harvey RC, Mullighan CG, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133:1548–59.
Barba P, Morgades M, Montesinos P, Gil C, Fox ML, Ciudad J, et al. Increased survival due to lower toxicity for high risk T-cell acute lymphoblastic leukemia patients in 2 consecutive pediatric-inspired PETHEMA trials. Eur J Haematol. 2019;102:79–86.
Modvig S, Madsen HO, Siitonen SM, Rosthoj S, Tierens A, Juvonen V, et al. Minimal residual disease quantification by flow cytometry provides reliable risk stratification in T-cell acute lymphoblastic leukemia. Leukemia. 2019;33:1324–36.
Albertsen BK, Grell K, Abrahamsson J, Lund B, Vettenranta K, Jonsson OG, et al. Intermittent versus continuous PEG-asparaginase to reduce asparaginase-associated toxicities: a NOPHO ALL2008 randomized study. J Clin Oncol. 2019; 37:1638–46.
Tulstrup M, Frandsen TL, Abrahamsson J, Lund B, Vettenranta K, Jonsson OG, et al. Individualized 6-mercaptopurine increments in consolidation treatment of childhood acute lymphoblastic leukemia: A NOPHO randomized controlled trial. Eur J Haematol. 2018;100:53–60.
Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10:147–56.
Uckun FM, Reaman G, Steinherez PG, Arthur DC, Sather H, Trigg M, et al. Improved clinical outcome for children with T-lineage acute lymphoblastic leukemia after contemporary chemotherapy: a Children’s Cancer Group Study. Leuk Lymphoma. 1996;24:57–70.
Goldberg JM, Silverman LB, Levy DE, Dalton VK, Gelber RD, Lehmann L, et al. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003;21:3616–22.
Amylon MD, Shuster J, Pullen J, Berard C, Link MP, Wharam M, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13:335–42.
Schmiegelow K, Forestier E, Hellebostad M, Heyman M, Kristinsson J, Soderhall S, et al. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia. 2010;24:345–54.
Asnafi V, Buzyn A, Thomas X, Huguet F, Vey N, Boiron JM, et al. Impact of TCR status and genotype on outcome in adult T-cell acute lymphoblastic leukemia: a LALA-94 study. Blood. 2005;105:3072–8.
Jain P, Kantarjian H, Jain N, Short NJ, Yin CC, Kanagal-Shamanna R, et al. Clinical characteristics and outcomes of previously untreated patients with adult onset T-ALL and T-lymphoblastic lymphoma (T-LL) with Hyper-CVAD based regimens. Am J Hematol. 2017;92:E595–7.
Durrant IJ, Prentice HG, Richards SM. Intensification of treatment for adults with acute lymphoblastic leukaemia: results of U.K. Medical Research Council randomized trial UKALL XA. Medical Research Council Working Party on leukaemia in adults. Br J Haematol. 1997;99:84–92.
Patel B, Kirkwood AA, Dey A, Marks DI, McMillan AK, Menne TF, et al. Pegylated-asparaginase during induction therapy for adult acute lymphoblastic leukaemia: toxicity data from the UKALL14 trial. Leukemia. 2017;31:58–64.
Tangen JM, Floisand Y, Haukas E, Naess IA, Skjelbakken T, Stapnes C, et al. Survival in adults with acute lymphoblastic leukaemia. Tidsskr Nor Laegeforen. 2010;130:1710–3.
Sakura T, Hayakawa F, Sugiura I, Murayama T, Imai K, Usui N, et al. High-dose methotrexate therapy significantly improved survival of adult acute lymphoblastic leukemia: a phase III study by JALSG. Leukemia. 2018;32:626–32.
Huguet F, Leguay T, Thomas X, Boissel N, Escoffre-Barbe M, Chevallier P. The upper age limit for a pediatric-inspired therapy in younger adults with Ph-negative acute lymphoblastic leukemia (ALL)? Analysis of the Graall-2005 study. San Diego: American Society of Haematology Congress; 2016.
Harrison CJ. Cytogenetics of paediatric and adolescent acute lymphoblastic leukaemia. Br J Haematol. 2009;144:147–56.
Acknowledgements
We thank all patients involved and clinicians assisting in ALL treatment and data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quist-Paulsen, P., Toft, N., Heyman, M. et al. T-cell acute lymphoblastic leukemia in patients 1–45 years treated with the pediatric NOPHO ALL2008 protocol. Leukemia 34, 347–357 (2020). https://doi.org/10.1038/s41375-019-0598-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0598-2
This article is cited by
-
Targeted therapy and immunotherapy for T cell acute lymphoblastic leukemia/lymphoma
Annals of Hematology (2023)
-
Effect of pediatric- versus adult-type chemotherapy regimens on outcomes of allogeneic hematopoietic stem cell transplants for adult T-cell acute lymphoblastic leukemia in first complete remission
Bone Marrow Transplantation (2022)
-
T cells targeted to TdT kill leukemic lymphoblasts while sparing normal lymphocytes
Nature Biotechnology (2022)
-
Predictive Value of Dynamic Peri-Transplantation MRD Assessed By MFC Either Alone or in Combination with Other Variables for Outcomes of Patients with T-Cell Acute Lymphoblastic Leukemia
Current Medical Science (2021)
-
Updated risk-oriented strategy for acute lymphoblastic leukemia in adult patients 18–65 years: NILG ALL 10/07
Blood Cancer Journal (2020)