Abstract
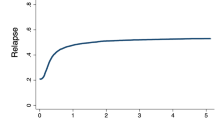
AML SCT-BFM 2007 was the first hematopoietic stem cell transplantation (HCT) trial in Germany to comply with the European Clinical Trials Directive, and aimed to standardize pediatric HCT for acute myeloid leukemia (AML) across centers in Germany, Austria, and the Czech Republic. Children with high-risk features and a good early response achieving a complete first remission (CR-1) and those in CR-2 after a first relapse were stratified to receive HCT from a matched donor after myeloablative conditioning consisting of busulfan, cyclophosphamide, and melphalan. Four-year EFS and OS were 61 and 70%. Cumulative incidence of relapse (CIR) was 22%. TRM was 15% and correlated with age reaching 9% (SE 3%) in children younger than 12 years and 31% (SE 9%) in older children and adolescents. Children with poorly responding primary disease or relapse were allocated to receive early HCT after a cytoreductive regimen with fludarabine, amsacrine, and cytarabine, followed by reduced intensity conditioning and prophylactic donor lymphocyte infusions. Four-year EFS and OS were 49 and 53%. CIR was 38% and TRM 11%. For patients with primary poor response disease, early use of RIC HCT followed by prophylactic DLI can induce long-term remissions in more than 50% (EFS 46% (SE 9%)).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
25 November 2019
A Correction to this paper has been published: https://doi.org/10.1038/s41375-019-0648-9
References
Ravindranath Y, Yeager AM, Chang MN, Steuber CP, Krischer J, Graham-Pole J, et al. Autologous bone marrow transplantation versus intensive consolidation chemotherapy for acute myeloid leukemia in childhood. Pediatric oncology group. N Engl J Med. 1996;334:1428–34.
Woods WG, Neudorf S, Gold S, Sanders J, Buckley JD, Barnard DR, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001;97:56–62.
Lee DH, Kwon YJ, Lim J, Kim Y, Han K, Chung NG, et al. Comparable outcomes of HLA-matched unrelated and HLA-identical sibling donor bone marrow transplantation for childhood acute myeloid leukemia in first remission. Pediatr Transplant. 2009;13:210–6.
Klusmann JH, Reinhardt D, Zimmermann M, Kremens B, Vormoor J, Dworzak M, et al. The role of matched sibling donor allogeneic stem cell transplantation in pediatric high-risk acute myeloid leukemia: results from the AML-BFM 98 study. Haematologica. 2012;97:21–9.
Niewerth D, Creutzig U, Bierings MB, Kaspers GJ. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116:2205–14.
Creutzig U, van den Heuvel-Eibrink MM, Gibson B, Dworzak MN, Adachi S, de Bont E, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120:3187–205.
Nguyen L, Fuller D, Lennon S, Leger F, Puozzo CIV. busulfan in pediatrics: a novel dosing to improve safety/efficacy for hematopoietic progenitor cell transplantation recipients. Bone Marrow Transplant. 2004;33:979–87.
Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70.
Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Prentice RL, Kalbfleisch JD, Peterson AV Jr., Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–54.
Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54.
Hasle H. A critical review of which children with acute myeloid leukaemia need stem cell procedures. Br J Haematol. 2014;166:23–33.
Creutzig U, Zimmermann M, Ritter J, Henze G, Graf N, Loffler H, et al. Definition of a standard-risk group in children with AML. Br J Haematol. 1999;104:630–9.
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74.
Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom medical research council trials. Blood. 2010;116:354–65.
Phillips GL, Shepherd JD, Barnett MJ, Lansdorp PM, Klingemann HG, Spinelli JJ, et al. Busulfan, cyclophosphamide, and melphalan conditioning for autologous bone marrow transplantation in hematologic malignancy. J Clin Oncol. 1991;9:1880–8.
Nevill TJ, Barnett MJ, Klingemann HG, Reece DE, Shepherd JD, Phillips GL. Regimen-related toxicity of a busulfan-cyclophosphamide conditioning regimen in 70 patients undergoing allogeneic bone marrow transplantation. J Clin Oncol. 1991;9:1224–32.
Strahm B, Nollke P, Zecca M, Korthof ET, Bierings M, Furlan I, et al. Hematopoietic stem cell transplantation for advanced myelodysplastic syndrome in children: results of the EWOG-MDS 98 study. Leukemia. 2011;25:455–62.
Pession A, Masetti R, Rizzari C, Putti MC, Casale F, Fagioli F, et al. Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood. 2013;122:170–8.
Beier R, Albert MH, Bader P, Borkhardt A, Creutzig U, Eyrich M, et al. Allo-SCT using BU, CY and melphalan for children with AML in second CR. Bone Marrow Transplant. 2013;48:651–6.
Locatelli F, Masetti R, Rondelli R, Zecca M, Fagioli F, Rovelli A, et al. Outcome of children with high-risk acute myeloid leukemia given autologous or allogeneic hematopoietic cell transplantation in the aieop AML-2002/01 study. Bone Marrow Transplant. 2015;50:181–8.
Baker KS, Bostrom B, DeFor T, Ramsay NK, Woods WG, Blazar BR. Busulfan pharmacokinetics do not predict relapse in acute myeloid leukemia. Bone Marrow Transplant. 2000;26:607–14.
McCune JS, Gooley T, Gibbs JP, Sanders JE, Petersdorf EW, Appelbaum FR, et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;30:167–73.
McCune JS, Gibbs JP, Slattery JT. Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet. 2000;39:155–65.
Ayala E, Figueroa J, Perkins J, Kim J, Yue B, Riches M, et al. Myeloablative intravenous pharmacokinetically targeted busulfan plus fludarabine as conditioning for allogeneic hematopoietic cell transplantation in patients with non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2015;15:335–40.
Parmar S, Rondon G, de Lima M, Thall P, Bassett R, Anderlini P, et al. Dose intensification of busulfan in the preparative regimen is associated with improved survival: a phase I/II controlled, randomized study. Biol Blood Marrow Transplant. 2013;19:474–80.
Andersson BS, Valdez BC, de Lima M, Wang X, Thall PF, Worth LL, et al. Clofarabine +/− fludarabine with once daily i.v. busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and MDS. Biol Blood Marrow Transpl. 2011;17:893–900.
Kebriaei P, Basset R, Ledesma C, Ciurea S, Parmar S, Shpall EJ, et al. Clofarabine combined with busulfan provides excellent disease control in adult patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1819–26.
Zhang H, Graiser M, Hutcherson DA, Dada MO, McMillan S, Ali Z, et al. Pharmacokinetic-directed high-dose busulfan combined with cyclophosphamide and etoposide results in predictable drug levels and durable long-term survival in lymphoma patients undergoing autologous stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1287–94.
Chen YB, Li S, Fisher DC, Driscoll J, Del Rio C, Abramson J, et al. Phase II trial of tandem high-dose chemotherapy with autologous stem cell transplantation followed by reduced-intensity allogeneic stem cell transplantation for patients with high-risk lymphoma. Biol Blood Marrow Transplant. 2015;21:1583–8.
Bitan M, He W, Zhang MJ, Abdel-Azim H, Ayas MF, Bielorai B, et al. Transplantation for children with acute myeloid leukemia: a comparison of outcomes with reduced intensity and myeloablative regimens. Blood. 2014;123:1615–20.
Gibson BE, Wheatley K, Hann IM, Stevens RF, Webb D, Hills RK, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–8.
Abrahamsson J, Forestier E, Heldrup J, Jahnukainen K, Jonsson OG, Lausen B, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29:310–5.
Kelly MJ, Horan JT, Alonzo TA, Eapen M, Gerbing RB, He W, et al. Comparable survival for pediatric acute myeloid leukemia with poor-risk cytogenetics following chemotherapy, matched related donor, or unrelated donor transplantation. Pediatr Blood Cancer. 2014;61:269–75.
Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5675–87.
Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, et al. Long term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108:1092–9.
de Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–72.
Feinstein LC, Sandmaier BM, Hegenbart U, McSweeney PA, Maloney DG, Gooley TA, et al. Non-myeloablative allografting from human leucocyte antigen-identical sibling donors for treatment of acute myeloid leukaemia in first complete remission. Br J Haematol. 2003;120:281–8.
Bunin NJ, Davies SM, Aplenc R, Camitta BM, DeSantes KB, Goyal RK, et al. Unrelated donor bone marrow transplantation for children with acute myeloid leukemia beyond first remission or refractory to chemotherapy. J Clin Oncol. 2008;26:4326–32.
Okamoto Y, Kudo K, Tabuchi K, Tomizawa D, Taga T, Goto H, et al. Hematopoietic stem-cell transplantation in children with refractory acute myeloid leukemia. Bone Marrow Transpl. 2019;54:1489–98.
O’Hare P, Lucchini G, Cummins M, Veys P, Potter M, Lawson S, et al. Allogeneic stem cell transplantation for refractory acute myeloid leukemia in pediatric patients: the UK experience. Bone Marrow Transpl. 2017;52:825–31.
Quarello P, Fagioli F, Basso G, Putti MC, Berger M, Luciani M, et al. Outcome of children with acute myeloid leukaemia (AML) experiencing primary induction failure in the AIEOP AML 2002/01 clinical trial. Br J Haematol. 2015;171:566–73.
Wareham NE, Heilmann C, Abrahamsson J, Forestier E, Gustafsson B, Ha SY, et al. Outcome of poor response paediatric AML using early SCT. Eur J Haematol. 2013;90:187–94.
Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29:1190–7.
Ivey A, Hills RK, Simpson MA, Jovanovic JV, Gilkes A, Grech A, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016;374:422–33.
Thol F, Gabdoulline R, Liebich A, Klement P, Schiller J, Kandziora C, et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood. 2018;132:1703–13.
Schuurhuis GJ, Heuser M, Freeman S, Bene MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131:1275–91.
Acknowledgements
This work was supported by grants from the German Federal Ministry of Education and Research (IFB-TX) (MGS), the Deutsche Forschungsgemeinschaft (Cluster of Excellence REBIRTH) (HL), Verein für krebskranke Kinder E.V. Hannover (MGS), and Neovii Biotech (formerly Fresenius) (MGS).
Author information
Authors and Affiliations
Contributions
MGS was principal investigator of the trial, designed the trial, collected and reviewed data, analyzed data, wrote and edited the manuscript; AB and RH were co-investigators of the trial, designed the trial, collected and reviewed data, wrote and edited the manuscript; HL and CPo legally sponsored the trial, verified and monitored data, wrote and edited the manuscript; KM collected data, analyzed data, and edited the manuscript; MZ designed the trial, did interim and final statistical analysis and created the figures; UC and DR coordinated the interface between upfront studies and the HCT trial, designed the trial, and edited the manuscript; PJL, MHA, PB, MKE, JG, BG, WH, TK, BK, CMK, RM, IM, CMN, CPe, CR, PGS, ASS, JS, PS, BS, WW, and RH, collected data and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MGS received research support from Neovii Biotech. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sauer, M.G., Lang, P.J., Albert, M.H. et al. Hematopoietic stem cell transplantation for children with acute myeloid leukemia—results of the AML SCT-BFM 2007 trial. Leukemia 34, 613–624 (2020). https://doi.org/10.1038/s41375-019-0584-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0584-8
This article is cited by
-
Reduced-toxicity myeloablative conditioning regimen using fludarabine and full doses of intravenous busulfan in pediatric patients not eligible for standard myeloablative conditioning regimens: Results of a multicenter prospective phase 2 trial
Bone Marrow Transplantation (2022)
-
Hematopoietic cell transplant in pediatric acute myeloid leukemia after similar upfront therapy; a comparison of conditioning regimens
Bone Marrow Transplantation (2021)
-
Treatment outcomes of pediatric acute myeloid leukemia: a retrospective analysis from 1996 to 2019 in Taiwan
Scientific Reports (2021)