Abstract
We compared patient-reported outcomes (PROs) with once-weekly carfilzomib 70 mg/m2 (Kd70 mg/m2) vs. twice-weekly carfilzomib 27 mg/m2 (Kd27 mg/m2) plus dexamethasone in relapsed or refractory multiple myeloma (RRMM). Patient-reported convenience/satisfaction collected at Cycle 2, Day 1 was compared between groups using logistic regression. European Organization for Research and Treatment of Cancer QOL Questionnaire (QLQ-C30), MM-module (QLQ-MY20), and EuroQoL-5 Dimensions-5 Levels (EQ-5D-5L) questionnaires were administered at baseline, then every other cycle. PROs were compared between groups using mixed models for repeated measures. Times from randomization to first deterioration (TTD) in scores were analyzed using Cox regression. PRO analyses included 469 patients. Once-weekly Kd70 mg/m2 patients reported greater convenience (odds ratio [OR], 4.98; p < 0.001) and satisfaction (OR, 2.41; p = 0.059) vs. twice-weekly Kd27 mg/m2. The mixed models for repeated measures demonstrated no clinically meaningful differences in scores between treatment arms. Clinically meaningful deterioration in QLQ-C30 Global Health Status/QOL rates were 34.2% (once-weekly Kd70 mg/m2) vs. 40.3% (twice-weekly Kd27 mg/m2). TTD was longer for once-weekly Kd70 mg/m2 vs. twice-weekly Kd27 mg/m2 for QLQ-C30 fatigue (HR, 0.79; p = 0.035), QLQ-MY20 disease symptoms (HR, 0.67; p = 0.008), EQ-5D-5L index score (HR, 0.58; p = 0.002), and EQ-5D-5L Visual Analog Scale (HR, 0.75, p = 0.031). Once-weekly Kd70 mg/m2 improved convenience/satisfaction, and reduced HRQOL deterioration vs. twice-weekly Kd27 mg/m2, supporting convenient, once-weekly Kd70 mg/m2 dosing in RRMM.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is the third most common hematologic malignancy worldwide [1], and primarily affects adults aged 65 years or older [2]. Patients with MM, including those with relapsed/refractory disease, often report significant impairment in health-related quality of life (HRQOL) due to disease-related symptoms like fatigue, pain, and reduced physical function [3,4,5]. Although many novel MM agents have shown improved efficacy and longer overall survival [6,7,8], evidence-based studies evaluating the impact of MM therapies on patients’ HRQOL have been limited [9]. HRQOL data has become increasingly important in guiding medical practice for MM [10, 11].
Carfilzomib is an irreversible proteasome inhibitor. The combination of carfilzomib (56 mg/m2) with dexamethasone has been approved for the treatment of relapsed or refractory MM (RRMM) [12]. Under this approval, carfilzomib is administered twice-weekly, on 2 consecutive days, intravenously (IV) over 30 min for 3 weeks of a 4-week cycle [12, 13]. Previous studies with bortezomib, a previous generation proteasome inhibitor, have demonstrated that MM patients treated with a once-weekly bortezomib dosing regimen were more adherent, had fewer dose reductions, and were treated for longer durations compared with patients on the twice-weekly schedule [14, 15]. Once-weekly dosing can substantially reduce patient travel and wait time, as well as caregiver time [16,17,18,19,20,21,22]. Improving the convenience of treatment is expected to improve HRQOL by allowing more time and opportunity for upholding social and familial roles [5]. Overall, a once-weekly Kd regimen may have value to patients beyond an improvement in treatment outcomes deriving from greater concordance with treatment regimens [23].
The phase III A.R.R.O.W. trial investigated the efficacy of once-weekly vs. twice-weekly Kd dosing schedules in patients with RRMM. A.R.R.O.W. demonstrated that once-weekly carfilzomib at 70 mg/m2 plus dexamethasone (Kd70 mg/m2) significantly improved progression-free survival (PFS) compared with twice-weekly carfilzomib at 27 mg/m2 plus dexamethasone (Kd27 mg/m2; median, 11.2 months vs. 7.6 months; hazard ratio, 0.69 (95% confidence interval (CI), 0.54–88); p = 0.0029) [23]. In addition, once-weekly Kd70 mg/m2 was associated with longer treatment exposure compared with twice-weekly Kd27 mg/m2, while exposure-adjusted discontinuation rates were similar between arms [23]. Thus, by increasing convenience with the utilization of the appropriate carfilzomib dose, A.R.R.O.W. demonstrated that patients stayed on once-weekly Kd70 mg/m2 longer and derived additional benefit without incremental risk compared with twice-weekly Kd27 mg/m2. Based on the results of the A.R.R.O.W. study, once-weekly Kd70 mg/m2 was recently approved for the treatment of patients with RRMM [12]. Patient-reported outcomes (PROs) were specified as exploratory endpoints in A.R.R.O.W.
Here we present patient-reported convenience and satisfaction and patient-reported HRQOL data of the once-weekly Kd70 mg/m2 and twice-weekly Kd27 mg/m2 treatment arms to assess from a patient perspective the impact of once-weekly Kd70 mg/m2 on convenience and quality of life (QOL). The following key subscales were prespecified for analysis: European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core Module (QLQ-C30) Global Health Status (GHS)/QOL, pain, fatigue, physical functioning, insomnia, and role functioning subscales; disease-specific MM questionnaire (QLQ-MY20) disease symptoms, side effects of treatment, and future perspective subscales; and European Quality of Life-5 Dimensions-5 Levels (EQ-5D-5L) index score and visual analog scale (VAS) score.
Materials and methods
Patients and study design
A.R.R.O.W. (Clinicaltrials.gov NCT02412878) was a randomized, open-label, phase III trial. Adult patients with RRMM who were previously treated with 2–3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, were included. Patients were recruited from approximately 118 sites across North America, Europe, and Asia. Full trial details have been published previously [23].
Eligible patients were randomized (1:1) to either once-weekly Kd70 mg/m2 or twice-weekly Kd27 mg/m2. Randomization was stratified according to International Staging System stage (1 vs. 2 or 3), refractoriness to bortezomib treatment (yes vs. no), and age (<65 years vs. ≥65 years). The once-weekly Kd70 mg/m2 arm received carfilzomib (30-min intravenous (IV) infusion) on days 1, 8, and 15 of all cycles (20 mg/m2 on day 1, cycle 1; 70 mg/m2 thereafter). The twice-weekly Kd27 mg/m2 arm received carfilzomib (10-min IV infusion) on days 1, 2, 8, 9, 15, and 16 (20 mg/m2 on days 1 and 2 during cycle 1; 27 mg/m2 thereafter). Dexamethasone at 40 mg was given to all patients on days 1, 8, 15 (all cycles), and 22 (cycles 1–9 only). Study treatment was administered in 28-day cycles, and cycles were repeated until disease progression, withdrawal of consent, or unacceptable toxicity.
The study protocol was approved by the institutional review boards or ethics committees of all participating institutions, and all patients provided written informed consent.
HRQOL assessments and endpoints
PROs were assessed with patient-reported convenience and satisfaction questionnaires, the EORTC QLQ-C30 [24], the EORTC QLQ-MY20 [25, 26], and the EQ-5D-5L index score and its VAS score [27]. Clinical outcome assessments were performed to measure these PROs. Patient-reported convenience and satisfaction with the carfilzomib dosing schedule were measured with two single-item 5-point Likert scales, with higher scores indicating a higher level of convenience/satisfaction. The validity, reliability, and relevance of the QLQ-C30 and QLQ-MY20 have been specifically demonstrated for patients with MM [26,27,28,29,30]. Further details regarding the subscales and scoring for the QLQ-C30, QLQ-MY20, and EQ-5D-5L are provided in the Supplemental Methods.
The minimal important difference (MID) represents the smallest clinically meaningful, group-level difference in a PRO score [31]. For the QLQ-C30, MIDs were prespecified in the statistical analysis plan based on the evidence-based interpretation guidelines for comparison between groups [32] and in accordance with others in the myeloma population [33] (Table 1). The QLQ-MY20 currently has no robust published estimates for MIDs; therefore, the standard error of measurement using Cronbach’s alpha at baseline was used as a proxy [34]. For the EQ-5D-5L analysis, a MID of 0.037 for the index score [35] and 7 for the VAS score [36] were used.
The responder definition is a threshold for defining meaningful change over time within an individual [37]. For the QLQ-C30 and QLQ-MY20, these were defined based on the minimal possible change on each scale, and the next increment as sensitivity analysis, in line with Cocks and Buchanan [38] (Table 1). As there are currently no separate recommendations for EQ-5D-5L, the MID was applied as a responder definition.
The QLQ-C30, QLQ-MY20, and EQ-5D-5L questionnaires were administered prior to study treatment on day 1 of cycle 1 (i.e., baseline), then every other cycle during treatment. For patients who discontinued therapy prior to progression, these questionnaires were administered during the follow-up period before disease progression every 12 weeks. Patient-reported convenience and satisfaction questionnaires were collected on day 1 of cycle 2 and end of treatment only.
Statistical analyses
The intent-to-treat population was used for analyses of missing data. The safety population, defined as all randomized patients who received at least one dose of carfilzomib or dexamethasone, was used for analyses of patient-reported convenience and satisfaction. All other analyses were based on the HRQOL analyses set, which included all randomized patients who completed at least one QLQ-C30, QLQ-MY20, EQ-5D-5L, or patient-reported convenience and satisfaction questionnaire.
Completion rates were calculated for all randomized patients at all HRQOL assessment visits, and for all patients expected to have an assessment (including randomized patients who were still in the study at that visit). HRQOL scores were plotted for each treatment arm to assess trends by missing data pattern.
At each assessment, the proportion of patients improved, stable, or worsened relative to baseline according to responder definitions was summarized for the QLQ-C30, QLQ-MY20, and EQ-5D-5L. Patient-reported convenience and satisfaction was compared between once-weekly Kd70 mg/m2 and twice-weekly Kd27 mg/m2 dosing schedules using multivariable binary and ordinal logistic regression adjusting for randomization stratification factors (Supplemental Materials).
A mixed model for repeated measures was used to compare PRO subscales between treatment arms. This primary model included treatment, continuous time, and randomization stratification factors as fixed effects, and a random intercept and slope. Baseline PRO scores were constrained to a common mean between treatments [39]. Least squares mean differences between treatment arms were estimated from the model. A sensitivity analysis tested the robustness of the primary mixed model for repeated measures to deviation from the missing at random assumption by jointly modeling longitudinal scores and time until last PRO assessment using a random effects association structure [40, 41]. In an additional post-hoc analysis, a treatment-by-time interaction was added to the primary mixed model for repeated measures as a fixed effect, allowing treatment effect to vary over time rather than forced constant. Least squares means by time point were plotted.
Time to deterioration (TTD; i.e., time from randomization until the first deterioration in PRO score meeting the responder definition corresponding to that score) was analyzed using a Cox regression analysis that accounted for the randomization stratification factors and baseline PRO scores. Hazard ratios were estimated from the stratified Cox regression model, p values from a stratified log rank test, and median TTD from unstratified Kaplan–Meier. Patients with no data or no baseline data were censored at the randomization date, and patients with no post-baseline data were censored at the randomization date +1 day.
Data sharing
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
Results
Patient population
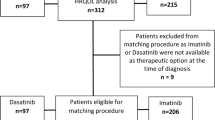
In the randomized phase III A.R.R.O.W. study, a total of 478 patients were randomized to treatment (once-weekly Kd70 mg/m2, n = 240; twice-weekly Kd27 mg/m2, n = 238) from September 9, 2015 to June 15, 2017. Among the randomized patients, a total of 469 had at least one post-baseline PRO assessment before end of treatment and were included in the PRO analyses (Fig. 1); once-weekly Kd70 mg/m2, n = 235; twice-weekly Kd27 mg/m2, n = 234). Baseline characteristics and mean baseline scores for the QLQ-C30, QLQ-MY20, and EQ-5D-5L subscales were similar between the once-weekly Kd70 mg/m2 and twice-weekly Kd27 mg/m2 arms (Table 2).
Patient disposition (CONSORT diagram) (The trial profile for the A.R.R.O.W. intent-to-treat population has been previously published [24]). EORTC QLQ-C30 European Organization for Research and Treatment of Cancer Quality of Life Core Module, EORTC QLQ-MY20 EORTC Quality of Life Multiple Myeloma Module 20, EQ-5D-DL European Quality of Life–5 Dimensions–5 Levels, Kd carfilzomib and dexamethasone, PRO patient-reported outcome
Completion and missing data patterns
QLQ-C30 completion rates at baseline were similar between the once-weekly Kd70 mg/m2 (90.4%) and twice-weekly Kd27 mg/m2 (92.9%) treatment arms (Table 3). Completion rates were higher in the once-weekly vs. twice weekly arm from cycle 9 onwards except at end of treatment. Similar completion rates were observed for the QLQ-MY20 and EQ-5D-5L questionnaires. For the convenience and satisfaction questions, completion rates were similar at cycle 2 day 1 and at end of treatment, respectively (once-weekly Kd70 mg/m2, 82.1 and 36.3%; twice-weekly Kd27 mg/m2, 81.9 and 44.1%). The proportion of patients completing the questionnaire (of those expected) were also similar between the arms. For the QLQ-C30, in the once-weekly Kd70 mg/m2 and twice-weekly Kd27 mg/m2 treatment arms, respectively, 76.7 and 79.4% of patients had a baseline and at least one post-baseline assessment; 5.8 and 3.4% had missing baseline assessment. Similar completion rates were observed for the QLQ-MY20 and EQ-5D-5L. Across the EORTC QLQ-C30 and EORTC QLQ-MY20 subscales and the EQ-5D-5L scores, there was no clear trend prior to dropout in either treatment arm for patients to be improving or declining.
QLQ-MY20 MID
Cronbach’s alpha for the QLQ-MY20 subscales were all above 0.7, indicating acceptable internal consistency [42]. The standard error of measurement was 9 for disease symptoms, 10 for future perspectives, and 7 for side effects of treatment. These standard errors of measurement were similar to those observed in previous studies [3, 43].
Descriptive analyses
The proportion of patients with improved scores from baseline was consistently higher (across most visits according to the two specified responder definitions) in the once-weekly Kd70 mg/m2 arm compared with the twice-weekly Kd27 mg/m2 arm in QLQ-C30 physical functioning, role functioning, and fatigue; QLQ-MY20 disease symptoms; and EQ-5D-5L VAS and index score. Other subscales exhibited no clear and consistent differences across the arms.
Patient-reported convenience and satisfaction
A greater proportion of patients in the once-weekly Kd70 mg/m2 vs. twice-weekly Kd27 mg/m2 arm reported convenience of the carfilzomib dosing schedule (very convenient/convenient/neutral; 82.4% vs. 65.8%). Additionally, patients in the once-weekly Kd70 mg/m2 arm reported greater convenience than patients in the twice-weekly Kd27 mg/m2 arm after adjustment for randomization stratification factors (OR, 4.98 (95% CI, 2.54–9.77); p < 0.001) for groupings of very convenient/convenient/neutral vs. inconvenient/very inconvenient. The once-weekly Kd70 mg/m2 regimen was also associated with higher odds of reporting convenience in ordinal logistic regression (OR, 3.03 (95% CI, 2.07–4.45); p < 0.001); results of other models are in Table 4.
The proportion of patients reporting satisfaction (very satisfied/satisfied/neutral) was numerically higher for the once-weekly Kd70 mg/m2 arm than the twice-weekly Kd27 mg/m2 arm (84.7% vs. 79.6%). This trend was also observed in a logistic regression model adjusting for randomization stratification factors for once-weekly Kd70 mg/m2 vs. twice-weekly Kd27 mg/m2 (OR, 2.41 [95% CI, 0.97–6.01]) for groupings of very satisfied/satisfied/neutral vs. dissatisfied/very dissatisfied. However, the once-weekly Kd70 mg/m2 regimen was associated with higher odds of reporting satisfaction in ordinal logistic regression, in which satisfaction was considered a categorical variable with five ordered values (very satisfied/satisfied/neutral/dissatisfied/very dissatisfied) (OR, 1.61 (95% CI, 1.10–2.36); p = 0.014); results of other models are in Table 4.
Treatment arm differences and TTD for QLQ-C30, QLQ-MY20, and EQ-5D-5L subscales
The primary mixed model for repeated measures did not demonstrate clinically meaningful differences in any scores. These findings were corroborated by the joint model. For all subscales of the QLQ-C30, QLQ-MY20, and EQ-5D-5L, the treatment-by-time interaction term two-sided p values were >0.05, indicating that treatment effect did not vary over time. When estimating mean differences between arms at each time point based on the mixed model for repeated measures with treatment-by-time interaction, the once-weekly Kd70 mg/m2 treatment arm showed clinically meaningful improvement in QLQ-C30 GHS/QOL scores at cycle 15 (Supplemental Fig. 1) and in the EQ-5D-5L index score at cycles 7, 9, 11, 13, and 15 (Supplemental Fig. 2). Improvements with once-weekly Kd70 mg/m2 vs. twice-weekly Kd27 mg/m2 were also estimated with a p value < 0.05 at multiple time points for QLQ-C30 GHS/QOL, fatigue, pain, physical functioning, insomnia, and role functioning; QLQ-MY20 disease symptoms; and EQ-5D-5L index and VAS scores; however, these differences were not clinically meaningful as they did not reach the prespecified MIDs (Supplemental Table 1; Supplemental Fig. 3).
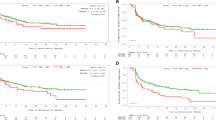
TTD was compared between treatment arms within each of the key subscales for QLQ-C30, QLQ-MY20, and EQ-5D-5L. There was a trend for longer median TTD for once-weekly Kd70 mg/m2 vs. twice-weekly Kd27 mg/m2 arms for QLQ-C30 fatigue (5.6 vs. 3.8 months; HR 0.79 (95% CI, 0.59–1.05), p = 0.035), QLQ-MY20 Disease Symptoms (14.8 vs. 7.4 months; HR 0.67 (95% CI, 0.48–94), p = 0.008), EQ-5D-5L index score (12.9 vs 5.6 months; HR 0.58 (95% CI, 0.42-80), p = 0.002), and EQ-5D-5L VAS score (median not reached vs. 7.4 months; HR, 0.75 (95% CI, 0.54–1.05); p = 0.031) (Fig. 2). In the once-weekly Kd70 mg/m2 and twice-weekly Kd27 mg/m2 arms, respectively, 34.2% and 40.3% of patients experienced a deterioration event of ≥5 points (responder definition) for the QLQ-C30 GHS/QOL subscale. Median (95% CI) TTD for GHS/QOL was 7.4 months (3.9–not estimable) in the once-weekly Kd70 mg/m2 arm vs. 3.8 months (3.7–5.8) in the twice-weekly Kd27 mg/m2 arm. TTD for GHS/QOL per Cox regression was similar between treatment arms (HR, 0.77 (95% CI, 0.57–1.04); p = 0.090). TTD was similar between the two treatment arms for the remaining key subscales of the QLQ-C30 and QLQ-MY20. Analyses based on a larger responder definition (Supplemental Table 1) demonstrated similar TTD between treatment arms for all key subscales.
Kaplan–Meier plots for time to first PRO deterioration. a EORTC QLQ-C30 Fatigue (Minimal important difference (MID) = 6). b EORTC MY-20 disease symptoms (MID = 9). c EQ-5D-5L utility index score (MID = 0.037). d EQ-5D-5L VAS score (MID = 7) EORTC QLQ-C30 European Organization for Research and Treatment of Cancer Quality of Life Core Module, EORTC QLQ-MY20 EORTC Quality of Life Multiple Myeloma Module 20, EQ-5D-DL European Quality of Life–5 Dimensions–5 Levels, Kd27 mg/m2 carfilzomib at 20/27 mg/m2 plus dexamethasone, Kd70 mg/m2 carfilzomib at 20/70 mg/m2 plus dexamethasone, PRO patient-reported outcome, VAS visual analog scale
Discussion
A.R.R.O.W. is the first study evaluating PROs with once-weekly, IV proteasome inhibitor dosing, and as such provides valuable information regarding HRQOL associated with this regimen. In the primary A.R.R.O.W. analysis, once-weekly Kd70 mg/m2 significantly prolonged PFS vs. twice-weekly Kd27 mg/m2 (median PFS, 11.2 months vs. 7.6 months; HR, 0.69 (95% CI, 0.54–83); p = 0.0029). Overall safety was comparable between groups, although the rate of grade ≥ 3 treatment-emergent adverse events (TEAEs) was higher in the once-weekly Kd70 mg/m2 (68%) vs twice-weekly Kd27 mg/m2 (62%) arm [23]. Patients in the once-weekly Kd70 mg/m2 arm had longer treatment exposure with similar rates of exposure-adjusted discontinuation compared with the twice-weekly Kd27 mg/m2 arm [23]. Our study complements these results by demonstrating that the value of once-weekly Kd70 mg/m2 dosing derives from delayed worsening of symptoms and greater convenience compared with twice-weekly Kd27 mg/m2, which may allow for more reliable, accurate, and durable concordance with the prescribed dosing regimen.
In our analysis, the overall differences between the once-weekly Kd70 mg/m2 and twice-weekly Kd27 mg/m2 arms did not reach clinical significance for any of the HRQOL key subscales when assessing mean scores over time in a mixed model for repeated measures. However, TTD in PRO scales was longer in the once-weekly Kd70 mg/m2 arm compared with the twice-weekly Kd27 mg/m2 arm for the QLQ-C30 fatigue, QLQ-MY20 disease symptoms, and EQ-5D-5L index and VAS scores. The longer TTD associated with once-weekly Kd70 mg/m2 might be correlated with the longer PFS in this arm, indicating that the achievement of PFS benefit can translate into longer maintenance of baseline QoL. In both treatment groups, median TTD for GHS/QOL was approximately 4 months shorter than median PFS, suggesting that PFS may overestimate the time of maintained QOL. In addition, after adjusting for randomization stratification factors, patients in the once-weekly Kd70 mg/m2 arm reported greater convenience and higher satisfaction than patients in the twice-weekly Kd27 mg/m2 arm, likely due to the less frequent dosing schedule with the Kd70 mg/m2 dose. By delaying HRQOL deterioration and improving convenience, the once-weekly administration of Kd70 mg/m2 can provide patients with a greater chance to participate in meaningful activities and to maintain a social role, both crucial factors in the HRQOL of MM patients [5].
We note that the higher incidence of grade ≥ 3 TEAEs in the once-weekly Kd70 mg/m2 arm in the primary A.R.R.O.W. analysis [23] did not translate into worse HRQOL in our study. The AE profile and QOL may not align because AEs and PRO instruments measure different aspects of the disease experience [44,45,46], and AEs are clinician-reported whereas our analysis focuses on PROs. Furthermore, the recall period of the questionnaires (1 week) differs from the more extensive nature of AE data collection.
Previous studies have shown that patients with RRMM generally have a high symptom burden and low HRQOL [3,4,5, 9]. Functional impairment from disease- and treatment-related symptoms, as well as the overall burden of a terminal diagnosis, can greatly affect HRQOL [3]. In a qualitative study of patients with relapsed MM who received bortezomib or thalidomide treatment, physical and cognitive functioning were reduced due to disease symptoms and treatment-related toxicity, with persistent peripheral neuropathy being considered especially burdensome [5]. HRQOL was reduced in these patients due to concerns about underlying disease, disability, and relapse.
As patients with MM are experiencing longer overall survival with novel agents [6,7,8], with 5-year relative survival rates now over 60% in patients under 70 years of age [7], improving quality of life has become an increasingly important treatment goal of MM therapy. Despite the complementary value of HRQOL when assessing efficacy and safety outcomes of novel MM agents, inconsistencies and weaknesses in HRQOL data analysis and presentation complicate the interpretation of treatment impact on HRQOL [10]. In a systematic review of HRQOL in longitudinal studies of patients with MM, HRQOL improvements were more likely to occur during first-line than in relapsed treatment regimens [11]. Thus, it is critical that MM therapies not only improve efficacy and have tolerable side effects, but also that HRQOL is improved or maintained, particularly in relapsed forms of MM.
Despite the clear association between MM symptoms and HRQOL, there remains a paucity of evidence-based data regarding the impact of MM therapies on symptom burden and HRQOL of patients with RRMM [9]. In general, proteasome inhibitor-containing regimens have been found to maintain or improve HRQOL in patients with RRMM compared with control or standard of care treatments [3, 47,48,49]. An HRQOL analysis of the landmark phase 3 ASPIRE trial found that carfilzomib in combination with lenalidomide and dexamethasone (KRd) improved clinical outcomes and GHS/QOL compared with Rd alone [3, 50, 51]. In the phase III ENDEAVOR trial, Kd56 mg/m2 was associated with superior clinical outcomes and improved GHS/QOL scores compared with bortezomib and dexamethasone (Vd) [52,53,54]. Patients treated with Kd56 mg/m2 also experienced slower TTD in GHS/QOL, physical and cognitive function, side effects, and neurotoxicity symptoms compared with Vd-treated patients in ENDEAVOR [54]. Our study is an important addition to the current body of literature on HRQOL in MM patients treated with carfilzomib, and builds upon the primary A.R.R.O.W. analysis efficacy and safety findings [24] by demonstrating that the increased convenience of the once-weekly regimen compared with twice-weekly regimen allowed patients to stay on therapy longer and thus produced superior clinical outcomes and prolonged HRQOL. We note that although dose per cycle administered for the once-weekly Kd70 mg/m2 arm is approximately one-third higher than that of the twice-weekly Kd27 mg/m2 control arm, the once-weekly Kd70 mg/m2 dose represents a 37.5% reduction over the standard approved twice-weekly Kd56 mg/m2 dose.
This study has some limitations. Patient-reported convenience and satisfaction were measured using individual items. Given the lack of published MIDs for the EORTC QLQ-MY20, a single distribution-based estimate was used. This needs confirmation in further studies using multiple anchors to estimate MID. As this was an open-label trial, patients were aware of the treatment they were receiving. Despite this, the completion rates for the convenience and satisfaction questions were similar across arms. However, completion rates, out of the patients expected to complete a questionnaire at each time point, for the other PRO measures were higher at later cycles in the once-weekly Kd70 mg/m2 arm. The findings from the mixed model were confirmed using a sensitivity analysis (joint model) and appear to be robust despite the differential dropout between treatment arms and high dropout particularly from cycle 17. There was no clear trend prior to dropout for patients to be either improving or declining in either of the treatment arms across the EORTC QLQ-C30, QLQ-MY20, and EQ-5D-5L. In addition, at this analysis stage, data for TTD have high levels of censoring (>50% for all subscales), and calculation of median TTD was not possible for all subscales. The PRO analyses were preplanned but not adjusted for multiplicity; nominal p values were provided for descriptive purposes. The depth of response may have correlated with PFS and time of maintained QOL in this study; however, this was not within the scope of the statistical analysis plan. We plan to further analyze this phenomenon in a future study.
In conclusion, this study demonstrated delayed disease symptom worsening, as well as greater patient-reported convenience and satisfaction, for the once-weekly Kd70 mg/m2 arm compared with the twice-weekly Kd27 mg/m2 arm. TTD was longer for the once-weekly Kd70 mg/m2 arm in fatigue, physical and role functioning, and health status. In the primary A.R.R.O.W. analysis, once-weekly Kd70 mg/m2 improved PFS over twice-weekly Kd27 mg/m2, with the incidence of adverse events being similar between the treatment groups [24]. Our findings complement the primary A.R.R.O.W. analysis by showing that these improved clinical outcomes led to prolonged HRQOL for the once-weekly regimen. Our results also add to the current knowledge of the efficacy, safety, and HRQOL of carfilzomib-based therapies in RRMM. These results build upon the ENDEAVOR and ASPIRE studies, which showed that carfilzomib-based regimens produced superior clinical outcomes which translated to improved and prolonged HRQOL compared with control regimens in RRMM [3, 50,51,52,53,54]. Collectively, the primary A.R.R.O.W. safety and efficacy data [24] and the current PRO analysis reinforce that once-weekly Kd70 mg/m2 dose is superior and convenient while delivering more favorable HRQOL than the commonly used Kd27 mg/m2 dose. Thus, once-weekly Kd70 mg/m2 should be considered an important alternative to twice-weekly Kd27 mg/m2 in clinical practice.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M et al. SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/ based on November 2017 SEER data submission, posted to the SEER web site, April 2018. Accessed 8 June 2018.
Stewart AK, Dimopoulos MA, Masszi T, Špička I, Oriol A, Hájek R, et al. Health-related quality of life results from the open-label, randomized, phase III ASPIRE trial evaluating carfilzomib, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in patients with relapsed multiple myeloma. J Clin Oncol. 2016;34:3921–30.
Leleu X, Kyriakou C, Vande Broek I, Murphy P, Bacon P, Lewis P, et al. Prospective longitudinal study on quality of life in relapsed/refractory multiple myeloma patients receiving second- or third-line lenalidomide or bortezomib treatment. Blood Cancer J. 2017;7:e543.
Mortensen GL, Salomo M. Quality of life in patients with multiple myeloma: a qualitative study. J Cancer Sci Ther. 2016;8:289–93.
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–8.
Turesson I, Bjorkholm M, Blimark CH, Kristinsson S, Velez R, Landgren O. Rapidly changing myeloma epidemiology in the general population: increased incidence, older patients, and longer survival. Eur J Haematol. 2018; https://doi.org/10.1111/ejh.13083
Thorsteinsdottir S, Dickman PW, Landgren O, Blimark C, Hultcrantz M, Turesson I et al. Dramatically improved survival in multiple myeloma patients in the recent decade: results from a Swedish population-based study. Haematologica 2018; https://doi.org/10.3324/haematol.2017.183475
Sparano F, Cavo M, Niscola P, Caravita T, Efficace F. Patient-reported outcomes in relapsed/refractory multiple myeloma: a systematic review. Support Care Cancer. 2018;26:2075–2090.
Sonneveld P, Verelst SG, Lewis P, Gray-Schopfer V, Hutchings A, Nixon A, et al. Review of health-related quality of life data in multiple myeloma patients treated with novel agents. Leukemia. 2013;27:1959–1969.
Nielsen LK, Jarden M, Andersen CL, Frederiksen H, Abildgaard N. A systematic review of health-related quality of life in longitudinal studies of myeloma patients. Eur J Haematol. 2017;99:3–17.
KYPROLIS® (carfilzomib) [prescribing information]: South San Francisco, CA: Onyx Pharmaceuticals, Inc.; 2018.
National Comprehensive Cancer Network (NCCN) 2018 guidelines. http://www.nccn.org/. Accessed October 30, 2018.
Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745–53.
Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Laumann K, et al. Once-versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood. 2010;115:3416–7.
Barrett-Lee P, Bloomfield D, Dougherty L, Harries M, Laing R, Patel H, et al. An audit to determine the time taken to administer intravenous bisphosphonate infusions in patients diagnosed with metastatic breast cancer to bone in a hospital setting. Curr Med Res Opin. 2007;23:1575–82.
Oglesby A, Sherif B, Odom D, Leahy M, Qian Y. Time and costs associated with preparing and administering zoledronic acid in patients with breast or prostate cancer and metastatic bone disease. Commun Oncol. 2009;6:494–502.
Schwartzberg LS, Stepanski EJ, Walker MS, Mathias S, Houts AC, Fortner BV. Implications of IV monoclonal antibody infusion reaction for the patient, caregiver, and practice: results of a multicenter study. Support Care Cancer. 2009;17:91–98.
Xie F, Hophins RB, Burke N, Habib M, Angelis CD, Pasetka M, et al. Time and labor costs associated with administration of intravenous bisphosphonates for breast or prostate cancer patients with metastatic bone disease: a time and motion study. Hosp Pract. 2014;42:38–45.
Richhariya A, Qian Y, Zhao Y, Chung K. Time associated with intravenous zoledronic acid administration in patients with breast or prostate cancer and bone metastasis. Cancer Manag Res. 2012;4:55–60.
Fortner B, Viale PH. Health economic analysis of the burden of infusion reactions on patients, caregivers, and provider. Oncology. 2009;23:31–36.
Chadda S, Larkin M, Jones C, Sykes D, Barber B, Zhao Z, et al. The impact of infusion reactions associated with monoclonal antibodies in metastatic colorectal cancer: a European perspective. J Oncol Pharm Pract. 2013;19:38–47.
Moreau P, Mateos M-V, Berenson JR, Weisel K, Lazzaro A, Song K, et al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018;19:953–64.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in onocology. J Natl Cancer Inst. 1993;85:365–76.
Stead ML, Brown JM, Velikova G, Kaasa S, Wisløff F, Child JA, et al. Development of an EORTC questionnaire module to be used in health-related quality-of-life assessment for patients with multiple myeloma. European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Br J Haematol. 1999;104:605–11.
Cocks K, Cohen D, Wisløff F, Sezer O, Lee S, Hippe E, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer. 2007;43:1670–8.
Devlin NJ, Shah KK, Feng Y, Mulhern B, Van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;27:7–22.
Wisløff F, Eika S, Hippe E, Holmberg E, Kaasa S, Palva I, et al. Measurement of health-related quality of life in multiple myeloma. Nordic Myeloma Study Group. Br J Haematol. 1996;92:604–13.
Leleu X, Petrucci MT, Welslau M, Broek IV, Murphy PT, Bottomley A, et al. Psychometric performance of the EORTC Quality-of-life core questionnaire (QLQ-C30) and QLQ-multiple myeloma (QLQ-MY20) in relapsed/refractory multiple myeloma (RRMM). Blood 2013;122:1721.
Osborne TR, Ramsenthaler C, de Wolf-Linder S, Schey SA, Siegert RJ, Edmonds PM, et al. Understanding what matters most to people with multiple myeloma: a qualitative study of views on quality of life. BMC Cancer. 2014;14:496.
Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10:S125–37.
Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011;29:89–96.
Delforge M, Dhawan R, Robinson D Jr, Meunier J, Regnault A, Esseltine DL, et al. Health-related quality of life in elderly, newly diagnosed multiple myeloma patients treated with VMP vs. MP: results from the VISTA trial. Eur J Haematol. 2012;89:16–27.
Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52:861–73.
McClure NS, Sayah FA, Xie F, Luo N, Johnson JA. Instrument-defined estimates of the minimally important difference for EQ-5D-5L index scores. Value Health. 2017;20:644–50.
Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharm Outcomes Res. 2011;11:163–9.
Cocks K, Buchanan J. Defining responders on the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (30-Item core module) (QLQ-C30) subscales. Qual Life Res. 2015;24:125.
Liang K-Y, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankyā. 2000;62:134–48.
Vonesh EF, Greene T, Schluchter MD. Shared parameter models for the joint analysis of longitudinal data and event times. Stat Med. 2006;25:143–63.
Fairclough DL. Design and analysis of quality of life studies in clinical trials, 3rd edn. Boca Raton, FL: CRC Press; 2010.
Nunnally J, Bernstein I. Psychometric theory. Mcgraw-Hill: New York, NY, 1994.
Dimopoulos MA, Delforge M, Hájek R, Kropff M, Petrucci MT, Lewis P, et al. Lenalidomide, melphalan, and prednisone, followed by lenalidomide maintenance, improves health-related quality of life in newly diagnosed multiple myeloma patients aged 65 years or older: results of a randomized phase III trial. Haematologica. 2013;98:784–8.
Atherton PJ, Watkins-Bruner DW, Gotay C, Moinpour CM, Satele DV, Winter KA, et al. The complementary nature of patient-reported outcomes (PROs) and adverse event reporting in cooperative group oncology clinical trial: a pooled analysis (NCCTG N0591). J Pain Symptom Manage. 2015;50:470–9.
Flores LT, Bennett AV, Law EB, Hajj C, Griffith MP, Goodman KA. Patient-reported outcomes vs. clinican symptom reporting during chemoradiation for rectal cancer. Gastrointest Cancer Res. 2012;5:119–24.
Basch E, Jia X, Heller G, Barz A, Sit L, Fruscione M, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101:1624–32.
Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–34.
Lee SJ, Richardson PG, Sonneveld P, Schuster MW, Irwin D, San Miguel JF, et al. Bortezomib is associated with better health-related quality of life than high-dose dexamethasone in patients with relapsed multiple myeloma: results from the APEX study. Br J Haematol. 2008;143:511–9.
Leleu X, Masszi T, Bahlis NJ, Viterbo L, Baker B, Gimsing P et al. Patient-reported health-related quality of life from the phase III TOURMALINE-MM1 study of ixazomib-lenalidomide-dexamethasone versus placebo-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma. Am J Hematol. 2018;93:981–3.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–52.
Siegel DS, Dimopoulos MA, Ludwig H, Facon T, Goldschmidt H, Jakubowiak A, et al. Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36:728–34.
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38.
Dimopoulos MA, Goldschmidt H, Niesvizky R, Joshua D, Chng WJ, Oriol A, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:1327–37.
Ludwig H, Moreau P, Dimopoulos MA, Mateos M-V, Kaiser MF, Hajek R, et al. Health-related quality of life in the ENDEAVOR study: carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed/refractory multiple myeloma. Blood Cancer J. 2019;9:23.
Acknowledgements
Medical writing and editorial assistance were provided by Sachi Yim, PhD, and Andrew Gomes, PhD, of BlueMomentum, an Ashfield Company, part of UDG Healthcare PLC, and funded by Amgen, Inc.
Funding
The A.R.R.O.W. study (Clinicaltrials.gov NCT02412878) was supported by Amgen, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PM reports honoraria from, and consulting or advisory roles for, Amgen, Celgene, Takeda, Janssen, BMS, Novartis, Millennium, and Onyx Pharmaceuticals. SK reports non-paid consulting and advisory board roles for AbbVie, Celgene, Janssen, Kite Pharma, Merck, and Takeda. RB reports researching funding from Amgen, Genentech, BMS, and AZ; speakers bureau roles for Amgen, Celgene, AZ, Genentech, and AbbVie; and consultancy roles for Pfizer and Sandoz. SI reports research funding from Ono, Takeda, Celgene, Janssen, Novartis, Chugai, Kyowa-Hakko Kirin, Bristol-Myers Squibb, MSD, Gilead, Daiiti Sankyo, Astellas, Toyama Chemical, Teijin Pharma, Abbie and Sanofi; and honoraria from Ono, Takeda, Celgene, Janssen, Bristol-Myers Squibb and Novartis. HG reports research support from Amgen, BMS, Celgene, Chugai, Janssen, Sanofi, Mundipharma, Takeda, and Novartis; advisory board roles for Adaptive Biotechnology, Amgen, BMS, Celgene, Janssen, Sanofi, and Takeda; and speakers bureau honoraria from ArtTempi, BMS, Celgene, Chugai, Janssen, and Novartis. KC reports employment with Adelphi Values; Consulting or advisory role fees from Orthox, Cardioprecision, Amgen, BMS, Endomag, BOC, Cognetivity, and Celgene; and travel and accommodations fees from BMS and Amgen. Andrew Trigg reports employment with Adelphi Values. AZ-K, EY, and SSP report employment with and stock holdings from Amgen. MD reports honoraria from, and advisory board roles for, Takeda, Celgene, Amgen, Janssen, and BMS.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moreau, P., Kumar, S., Boccia, R. et al. Convenience, satisfaction, health-related quality of life of once-weekly 70 mg/m2 vs. twice-weekly 27 mg/m2 carfilzomib (randomized A.R.R.O.W. study). Leukemia 33, 2934–2946 (2019). https://doi.org/10.1038/s41375-019-0480-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0480-2
This article is cited by
-
Use of the European Organisation for Research and Treatment of Cancer multiple myeloma module (EORTC QLQ-MY20): a review of the literature 25 years after development
Blood Cancer Journal (2023)
-
The impact of current therapeutic options on the health-related quality of life of patients with relapse/refractory multiple myeloma: a systematic review of clinical studies
Journal of Cancer Survivorship (2023)
-
Mechanistic Pharmacokinetic/Pharmacodynamic Modeling in Support of a Patient-Convenient, Longer Dosing Interval for Carfilzomib, a Covalent Inhibitor of the Proteasome
Clinical Pharmacokinetics (2023)
-
Once-weekly vs. twice-weekly carfilzomib dosing in a subgroup of Japanese relapsed and refractory multiple myeloma patients from a randomized phase 3 trial (A.R.R.O.W.) and comparison with ENDEAVOR
International Journal of Hematology (2021)
-
Quality of life analyses in patients with multiple myeloma: results from the Selinexor (KPT-330) Treatment of Refractory Myeloma (STORM) phase 2b study
BMC Cancer (2021)