Abstract
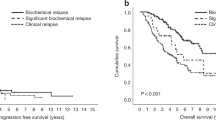
Achieving a complete response (CR) is associated with improved overall survival (OS) in multiple myeloma (MM), but data on duration of CR (DurCR) are limited. We evaluated 351 patients (2004–2016), achieving CR with first-line therapy. Patients with sustained DurCR ≥ 24 months (n = 177) had better OS; 150 vs. 81 months, p < 0.001. DurCR ≥ 24 months remained a significant predictor for OS (HR: 0.3, 95% CI: 0.2–0.5, p < 0.001) after adjusting for age, revised ISS stage, transplant and maintenance therapy. Landmark analysis at 24 months demonstrated similar results, OS: 150 vs. 83 months, p < 0.001. Survival benefit persisted even after loss of CR, with median OS being 89 vs. 56 months (p = 0.005), respectively. Patterns of loss of CR were heterogeneous, with biochemical relapse in 59 (25%); symptomatic relapse in 58 (24%); positive immunofixation/monoclonal protein rise not meeting relapse/progression criteria in 88 (37%) and abnormal free light chain ratio in LC MM in 34 (14%) patients. OS from start of first-line therapy was superior in patients starting second-line treatment for biochemical vs. symptomatic relapse (125 vs. 81 months, p = 0.001). This is likely attributable to underlying disease biology and prevention of end-organ damage by early treatment initiation, as benefit was independent of R-ISS stage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fulciniti M, Munshi NC, Martinez-Lopez J. Deep response in multiple myeloma: a critical review. Biomed Res Int. 2015;2015:832049.
Gay F, Larocca A, Wijermans P, Cavallo F, Rossi D, Schaafsma R, et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood. 2011;117:3025–31.
Harousseau JL, Dimopoulos MA, Wang M, Corso A, Chen C, Attal M, et al. Better quality of response to lenalidomide plus dexamethasone is associated with improved clinical outcomes in patients with relapsed or refractory multiple myeloma. Haematologica. 2010;95:1738–44.
Lahuerta J-J, Paiva B, Vidriales M-B, Cordón L, Cedena M-T, Puig N, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35:2900–10.
Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol. 2017;3:28–35.
van de Velde HJ, Liu X, Chen G, Cakana A, Deraedt W, Bayssas M. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92:1399–406.
Avet-Loiseau H, Corre J, Lauwers-Cances V, Chretien M-L, Robillard N, Leleu X, et al. Evaluation of minimal residual disease (MRD) by next generation sequencing (NGS) is highly predictive of progression-free survival in the IFM/DFCI 2009 Trial. Blood. 2015;126:191. -
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Gonsalves WI, Rajkumar SV, Gertz MA, Dispenzieri A, Lacy MQ, Buadi FK, et al. Clinical course and outcomes of patients with multiple myeloma who relapse after autologous stem cell therapy. Bone Marrow Transplant. 2016;51:1156–8.
Kumar S, Mahmood ST, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Impact of early relapse after auto-SCT for multiple myeloma. Bone Marrow Transplant. 2008;42:413–20.
Kumar SK, Dispenzieri A, Fraser R, Mingwei F, Akpek G, Cornell R, et al. Early relapse after autologous hematopoietic cell transplantation remains a poor prognostic factor in multiple myeloma but outcomes have improved over time. Leukemia. 2017;32:986–95.
Majithia N, Rajkumar SV, Lacy MQ, Buadi FK, Dispenzieri A, Gertz MA, et al. Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents. Leukemia. 2016;30:2208–13.
Barlogie B, Anaissie E, Haessler J, van Rhee F, Pineda-Roman M, Hollmig K, et al. Complete remission sustained 3 years from treatment initiation is a powerful surrogate for extended survival in multiple myeloma. Cancer . 2008;113:355–9.
Chim CS, Liu H, Lie AK, Chan EY, Ho S, Wong M, et al. Unsustained complete response of less than 24 months after autologous stem cell transplantation predicts aggressive myeloma with short survival. Hematol Oncol. 2014;32:205–11.
Lehners N, Becker N, Benner A, Pritsch M, Lopprich M, Mai EK, et al. Analysis of long-term survival in multiple myeloma after first-line autologous stem cell transplantation: impact of clinical risk factors and sustained response. Cancer Med. 2018;7:307–16.
Hoering A, Crowley J, Shaughnessy JD, Hollmig K, Alsayed Y, Szymonifka J, et al. Complete remission in multiple myeloma examined as time-dependent variable in terms of both onset and duration in Total Therapy protocols. Blood. 2009;114:1299–305.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9.
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–5.
Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101–19.
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised International Staging System for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9.
Greipp PR, Miguel JS, Durie BGM, Crowley JJ, Barlogie B, Bladé J, et al. International Staging System for Multiple Myeloma. J Clin Oncol. 2005;23:3412–20.
JMP®, Version 12. SAS Institute Inc., Cary, NC, 1989–2007.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Cox DR. Regression models and life-tables. J R Stat Soc Ser B (Methodol). 1972;34:187–220.
Barlogie B, Mitchell A, van Rhee F, Epstein J, Morgan GJ, Crowley J. Curing myeloma at last: defining criteria and providing the evidence. Blood. 2014;124:3043–51.
Author contributions:
SS and SKK designed the study, collected and analyzed data and wrote the manuscript. NT collected the data and contributed to writing and critically appraising the manuscript. AD, MAG, FKB, MQL, DD, ALF, SRH, MAH, WIG, RMW, TK, YLH, PK, RAK, NL, RSG, SVR contributed to writing and critically appraising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest directly related to this work. Below is a list of all financial disclosures for authors: SS: Honoraria for advisory board, Janssen AD: Research Funding from Celgene, Takeda, Prothena, Jannsen, Pfizer, Alnylam, GSKPK: Research Funding from Celgene, Takeda MQL: Research Funding from Celgene MAG: Honoraria/consultancy from Ionis, Alnylam, Prothena, Celgene, Janssen, Specytrum, Annexon, Apellis, Amgen, Medscape, Abbvie, Research to Practice, Physcians Education Resource and Teva; SKK: Research Funding and membership on an entity’s Board of Directors or advisory committees: AbbVie, Celgene, Janssen, KITE, Merck. Membership on an entity's Board of Directors or advisory committees: Oncopeptides, Takeda. Research funding from Novartis and Roche.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sidana, S., Tandon, N., Dispenzieri, A. et al. Relapse after complete response in newly diagnosed multiple myeloma: implications of duration of response and patterns of relapse. Leukemia 33, 730–738 (2019). https://doi.org/10.1038/s41375-018-0271-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0271-1
This article is cited by
-
Gaps and opportunities in the treatment of relapsed-refractory multiple myeloma: Consensus recommendations of the NCI Multiple Myeloma Steering Committee
Blood Cancer Journal (2022)
-
Management of Multiple Myeloma in the Middle East: Unmet Needs, Challenges and Perspective
Clinical Hematology International (2022)
-
Minimal residual disease in multiple myeloma: current status
Biomarker Research (2021)
-
The impact of re-induction prior to salvage autologous stem cell transplantation in multiple myeloma
Bone Marrow Transplantation (2019)