Abstract
Objective
To determine the relationship between interventricular septal position (SP) and right ventricular systolic pressure (RVSP) and mortality in infants with severe BPD (sBPD).
Study design
Infants with sBPD in the Children’s Hospitals Neonatal Database who had echocardiograms 34–44 weeks’ postmenstrual age (PMA) were included. SP and RVSP were categorized normal, abnormal (flattened/bowed SP or RVSP > 40 mmHg) or missing.
Results
Of 1157 infants, 115 infants (10%) died. Abnormal SP or RVSP increased mortality (SP 19% vs. 8% normal/missing, RVSP 20% vs. 9% normal/missing, both p < 0.01) in unadjusted and multivariable models, adjusted for significant covariates (SP OR 1.9, 95% CI 1.2–3.0; RVSP OR 2.2, 95% CI 1.1–4.7). Abnormal parameters had high specificity (SP 82%; RVSP 94%), and negative predictive value (SP 94%, NPV 91%) for mortality.
Conclusions
Abnormal SP or RVSP is independently associated with mortality in sBPD infants. Negative predictive values distinguish infants most likely to survive.
Similar content being viewed by others
Introduction
Severe bronchopulmonary dysplasia (sBPD) affects approximately 20% of extremely low-birthweight infants [1]. Pulmonary arterial hypertension (PH) can occur in 15–25% of infants with sBPD, and is associated with an increased risk of mortality [2,3,4]. Recognizing this concern, the American Heart Association recommends screening echocardiography to monitor for PH in infants with BPD [5]. However, echocardiographic parameters are limited by subjective, varied, or missing measures, as well as modest correlation with pulmonary arterial pressure measured via cardiac catheterization [6,7,8,9]. Further, screening for PH often varies from center to center. Understanding whether standard clinically used echocardiographic parameters of PH are associated with mortality would help to inform clinical practices with respect to screening infants with sBPD for PH.
Our objective was to determine whether interventricular septal position and right ventricular systolic pressure on echocardiography performed between 34 and 44 weeks’ post-menstrual age (PMA) were associated with in-hospital mortality in infants with sBPD. We hypothesized that these measures could be used to predict inpatient mortality in a real-world clinical cohort.
Methods
We performed a secondary analysis of data from the Children’s Hospitals Neonatal Database (CHND), which includes prospectively collected data on all infants admitted to participating Level IV neonatal intensive care units (NICUs) in the United States and Canada. Infant clinical data were collected by trained chart abstractors at each site, with periodic assessments of inter-rater agreement scores [10]. De-identified data analyses were approved by the Institutional Review Board of Stanley Manne Research Institute affiliated with the Ann and Robert H. Lurie Children’s Hospital of Chicago. All participating centers obtained regulatory oversight to participate in the CHND registry.
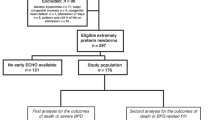
We included infants born <32 weeks’ gestation who were admitted to a participating NICU between 2010 and 2015, who had sBPD, and who had at least one echocardiogram between 34 and 44 weeks’ PMA. sBPD was defined as receiving positive pressure ventilation, >2 L/min flow by nasal cannula, or fractional inspired oxygen (FiO2) > 0.3 at or after 36 weeks’ PMA [2, 10, 11]. Infants were excluded if they died prior to 36 weeks’ PMA, were transferred out of the participating hospital before discharge, or if they had major congenital anomalies. Infants with an isolated atrial septal defect, ventricular septal defect, or patent ductus arteriosus were retained in the study.
The primary outcome was mortality between 36 weeks’ PMA and discharge from a CHND hospital. Primary exposures of interest were echocardiographic measures of interventricular septal position (SP) and right ventricular systolic pressure (RVSP) recorded between 34 and 44 weeks’ PMA. SP was defined as abnormal if there was documentation of septal position being flattened or bowed to the left. RVSP was categorized as abnormal if the result was >40 mmHg; this cut-off was determined a priori corresponding to values >40 mmHg being more than half of the systemic blood pressure [7, 12, 13]. For infants with multiple echocardiograms, we examined all reports occurring between 34 and 44 weeks PMA; we categorized the infant as having an abnormal echocardiographic parameter if any available studies had an abnormal parameter. If no available echocardiography reports documented RVSP or SP, we categorized the data as missing and retained that infant in the dataset. Direction of flow across the patent ductus arteriosus (PDA) and the interatrial septum were not considered as exposures for this study because the PDA was closed and interatrial septal flow was minimal in nearly all subjects between 34 and 44 weeks’ PMA. Instead, PDA ligation and interatrial communication were included as potential covariates.
We included other clinical covariates available in the CHND that we hypothesized might confound the relationship between echocardiographic parameters and mortality. Maternal characteristics included were race, diabetes, hypertension, chorioamnionitis, multiple gestation, and receipt of antenatal steroids. Neonatal characteristics included gestational age, birthweight, small for gestational age (defined as sex-specific ≤ 10th percentile of birthweight for gestational age) [14], duration of mechanical ventilation in the CHND nursery, receipt of mechanical ventilation at 36 weeks’ PMA, receipt of systemic corticosteroids, bloodstream infections, atrial septal communications, or PDA ligation. A respiratory severity score was also calculated as a product of the mean airway pressure and the fractional oxygen requirement (MAP × FiO2) at 36 weeks’ PMA and compared between groups (see Table 1). Bloodstream infections were defined as any confirmed bacterial or fungal organisms recovered from a blood culture during the hospital admission. Necrotizing enterocolitis was defined as any of the following conditions: (1) Surgical NEC; (2) NEC at post-mortem examination or (3) if both clinical symptoms (bilious aspirate or emesis, abdominal distention, and occult/gross blood in stool) and radiographic findings (pneumotosis intestinalis, hepatobiliary gas or pneumoperitonem) were present. These findings correspond to a NEC Stage IIb or higher. Airway comorbidities were defined as laryngeal, tracheal, or bronchial malacia, laryngeal or tracheal stenosis, or vocal cord paralysis or paresis.
Statistical analysis
We first assessed the proportion of echocardiographic parameters that were normal, abnormal and missing in the study cohort. We compared the clinical characteristics by SP and RVSP groups of normal, abnormal and missing, using chi-squared or Fisher’s exact tests for differences in proportion and Kruskal−Wallis nonparametric tests for differences of medians. For the unadjusted association between echocardiographic parameters and mortality, we calculated the sensitivity, specificity, positive and negative predictive value, and relative risk. Next, all covariates with bivariate association of p < 0.05 were entered into multilevel multivariable logistic regression models with center as a random intercept. Because we assumed the echocardiographic exposure variables to be collinear, we ran two separate models, one with septal position alone as the exposure, and one with RVSP alone as the exposure. For each model, abnormal and missing echocardiographic parameters were each tested against a referent group of infants with normal echocardiographic parameters. Statistical significance was defined as p value < 0.05. SAS v 9.2 (SAS Institute, Cary, North Carolina) was used for analyses.
Results
The database included 12,221 infants born <32 weeks’ gestation who were alive at 36 weeks’ PMA and who did not have congenital anomalies. Of those, 2806 infants had sBPD and 1412 (50%) infants with sBPD had one or more echocardiograms between 34 and 44 weeks’ PMA. We excluded 255 infants who were transferred to another hospital before discharge. This resulted in a study cohort of 1157 infants; 1042 (90%) survived to hospital discharge, while 115 (10%) died. Infants who died had a later PMA at admission to the NICU (median 31 days [IQR 27–38] vs. 28 [IQR 26–33], p < 0.001), lower birthweight (median 685 g [IQR 560–840] vs. 748 g [IQR 620–910], p = 0.001), were on the ventilator longer (median 102 days [IQR 51–144] vs. 42 days [IQR 19–71], p < 0.001), and were more likely to have received systemic corticosteroids (69% vs. 38%, p < 0.001) or to have received a diagnosis of necrotizing enterocolitis (22% vs. 10%, p = 0.001) than infants who survived. The cause of death in our cohort was primarily respiratory failure (67%, n = 77/115), followed by multiorgan system failure (16%, n = 18/115). Forty-six infants (60%, n = 46/77) who died of respiratory causes also had a clinical diagnosis of pulmonary hypertension. Less common causes of death were intra-abdominal catastrophe (4%), central nervous system injury (3%), infection (3%), renal failure (3%), and other causes (4%). Infants with abnormal septal position and RVSP had a median of 2 echocardiograms, and infants with normal or missing parameters had a median of one echocardiogram during their neonatal hospitalization. As shown in Table 1, infants with abnormal RVSP measurements had echocardiograms performed at a slightly later PMA those with normal measurements (34 weeks vs. 30 weeks), and the median PMA of echocardiogram attainment in infants with abnormal and normal septal position was 31 weeks.
Septal position (SP) was categorized as normal in 616 (53%) infants, abnormal in 227 (20%) infants, and missing in 314 (27%) of infants. Table 1 (top) compares characteristics of infants by SP. SP groups differed with respect to gestational age and birthweight, age at referral to CHND NICU, birthweight < 10th percentile for gestational age, maternal race, duration of mechanical ventilation, respiratory severity score at 36 weeks’ PMA, receipt of systemic corticosteroids, bloodstream infections, and atrial septal defects. The proportion of infants receiving PDA ligation was similar between groups but the PMA at PDA ligation was later in infants with abnormal SP. Infants with missing SP had clinical characteristics that were similar to those of infants with normal position. RVSP was categorized as normal in 230 (20%) infants, abnormal in 84 (7%), and missing in 843 (73%). Table 1 (bottom) compares characteristics of infants by RVSP. RVSP categories differed in categories of birthweight, sex, maternal race, singleton birth and atrial septal defects. Rates of PDA ligation were similar between groups but the median PMA at ligation was later in infants with abnormal RVSP. Infants with missing RVSP had the largest birthweight, the lowest receipt of postnatal systemic steroids, and the lowest proportion of atrial septal defects.
Abnormal SP and RVSP were each associated with higher odds of mortality (Table 2, top). In unadjusted analysis, abnormal SP was associated with 2.6-fold increase in mortality, and after adjustment for significant covariates, SP was associated with 1.9-times the odds of mortality, when compared with infants with normal SP (p = 0.01). However, infants with missing SP did not have significantly different odds of mortality from those with normal SP. Receiving ventilation at 36 weeks’ PMA, having a bloodstream infection, receiving postnatal steroids, and being admitted at a later PMA were also significant risk factors for mortality in both unadjusted and adjusted SP models. Similarly, in both unadjusted models and adjusted models, abnormal RVSP was associated with a 2.2-fold increase in mortality (p = 0.02), whereas infants with missing RVSP data had mortality rates that were similar to infants with normal RVSP values (Table 2, bottom). Ventilation at 36 wks’ PMA, bloodstream infection, postnatal steroid use, and admission PMA remained significantly associated with mortality in adjusted RVSP models. Whereas atrial septal defects marginally increased the odds of mortality in SP models, the presence of an interatrial communication significantly increased mortality by 1.9-fold in RVSP models.
Overall, infants with abnormal septal position had a mortality rate that was 19% compared with 8% of infants with normal SP, and 8% for infants with normal/missing data (abnormal SP n = 43/227 vs. normal SP n = 50/616 or normal/missing SP n = 72/930). Mortality for infants with abnormal RVSP was 20% (n = 17/84) compared with 10% for infants with normal data (n = 24/230) and 9% for infant with normal/missing data (n = 98/1073).
Test characteristics of the abnormal echocardiographic parameters are shown in Table 3. When comparing infants with abnormal SP or RVSP to those with normal parameters, these parameters were highly specific (82% and 94%, respectively) but poorly sensitive (37% and 15%) markers of mortality. The negative predictive value was over 90% for each parameter. This pattern was nearly identical when comparing abnormal SP or RVSP to infants either a normal or missing parameter.
Discussion
In this multicenter clinical cohort of infants with sBPD, we found that septal position and RVSP > 40 mmHg were significantly associated with in-hospital mortality after adjusting for significant clinical characteristics. We also found that infants with missing documentation of RVSP and/or septal position had clinical and mortality outcomes similar to those with documented normal echocardiographic parameters.
Pulmonary hypertension is a significant contributor to morbidity and mortality in premature infants with BPD [2, 15,16,17,18]. In 2015, the American Heart Association recommended echocardiographic screening of infants with moderate or severe BPD beginning at 36 weeks PMA with serial echocardiography thereafter [5]. Compared to cardiac catheterization, echocardiographic screening for PH has been limited in its ability to detect and grade the severity of pulmonary hypertension [7]. We found that infants with documented abnormal RVSP or septal position had approximately double the odds of mortality, compared to infants with normal echocardiographic parameters. This association persisted even after adjusting for other significant predictors of mortality including gestational age, receipt of mechanical ventilation at 36 weeks’ PMA, atrial septal defect, bloodstream infection, postnatal corticosteroid use, and PMA at admission to the participating center. These results demonstrate that even among sick neonates with severe BPD, abnormal SP and RVSP are independent predictors of mortality. In that context, our results demonstrating the potential impact of real-world echocardiographic data provide further support for adherence to the new guidelines.
When we compared infants with missing echocardiographic data to infants with normal parameters for septal position, we found that infants with missing data had an odds of mortality that was similar to infants with normal echocardiographic parameters. Mourani et al. found that the sensitivity and positive-predictive value of echocardiographic parameters for the detection of PH is enhanced when tricuspid regurgitant jet velocity can be measured as a proxy for pulmonary artery pressure [7]. Unfortunately, several studies have noted the challenge of obtaining tricuspid regurgitant jet velocity measurements, even when echocardiography is being performed in accordance with prespecified research protocols and on older pediatric patients [19, 20]. Indeed, in our cohort, tricuspid regurgitant jet velocity and resultant right ventricular systolic pressure was recorded in only 27% of echocardiographic reports, highlighting the practical limitations of obtaining this important variable at the bedside. Septal position was more commonly documented in our cohort, with 73% of echocardiographic reports having documentation of septal position. Assignment of septal position may be subject to considerable subjectivity and interobserver variability [21]. More quantitative measures of septal position, such as eccentricity index, are now being investigated in infants with BPD and hold promise as a means of standardizing the echocardiographic approach [19, 22]. Nonetheless, our finding that infants with missing echocardiographic data had mortality rates similar to infants with documented normal echocardiographic parameters suggests that the inherent difficulties of obtaining standard echocardiographic measurements should not prevent efforts at screening. Further, echocardiography correctly identified infants who were likely to survive, as shown by the high specificity and negative predictive values of both septal flattening and RVSP in our study. As such, septal position and RVSP measures on echocardiography between 34 and 44 weeks’ PMA can be helpful in delineating risk status for in-hospital mortality in infants with sBPD.
Surprisingly, only half of our initial cohort of infants with severe BPD had screening echocardiography between 34 and 44 weeks’ PMA. This finding is in keeping with recent surveys of neonatal members of the American Academy of Pediatrics. This survey found that 83% of physicians would obtain an echocardiogram at 36 weeks’ corrected gestational age for infants with moderate or severe BPD, but only 46% of respondents had a formal screening program in place at their institution that facilitated the implementation of these guidelines [23]. Our study cohort occurred prior to the publication of the recent American Heart Association screening guidelines; future work, including our observations in this study, should serve to increase the rate of screening for PH in infants with BPD. Quality initiatives to standardize the approach to PH screening in infants with BPD across sites are now being developed. Even as screening efforts advance, we acknowledge that data are limited on whether early detection and pharmacologic treatment of pulmonary hypertension in infants with severe BPD improves survival. Retrospective evaluations of this association may be plagued with concerns about confounding by indication, and the feasibility and cost of randomized controlled trials can be prohibitive. Additionally, the natural course of milder forms of pulmonary hypertension is to resolve without pharmacologic therapy [24, 25]. However, identifying the patients at risk for poor outcomes is essential in the neonatal period to allow appropriate risk categorization and follow-up after hospital discharge. Overall, one in four infants with severe BPD may develop pulmonary hypertension, but 41−62% will be diagnosed after hospital discharge and will experience morbidity and mortality in the first year of life as a result of their diagnosis [1, 2, 24, 26]. As such, even while the efficacy of pharmacologic therapy for pulmonary hypertension is being evaluated, echocardiographic screening for infants with severe BPD may allow proven interventions such as aggressive management of respiratory acidosis, prevention of intermittent hypoxia, targeting of optimal nutrition and feeding modes, and engagement of multidisciplinary care to be employed early and aggressively in order to improve outcomes for high-risk infants [1, 27,28,29,30,31].
Our study has important limitations. Our cohort was derived from a referral-based, quaternary care population and may not be generalizable to other cohorts. We further recognize that the infants in our cohort had severe BPD, and although controlling for the need for mechanical ventilation at 36 weeks’ post-menstrual age may have adjusted for the sickest patients, it is possible that associations between echocardiographic parameters and mortality may differ in infants with less severe lung disease. The echocardiograms in our study were performed at clinical discretion, which may lead to some ascertainment and selection bias in our sample.
In conclusion, we have shown that abnormalities in echocardiographic parameters, such as septal flattening and RVSP, are associated with nearly a two-fold increase in the odds of mortality prior to hospital discharge in infants with severe BPD. This association persists after controlling for important clinical variables. Further, infants with normal parameters are more likely to survive their initial hospitalization than those with abnormal values in our cohort. These findings support the recommendation to implement pulmonary hypertension screening for infants with severe BPD despite the known limitations of echocardiography in a neonatal population. Future quality improvement initiatives that define the timing and standardize the optimal approach to echocardiography in this high-risk population are warranted.
Code availability
The computer code used to generate statistical analyses may be made available upon request to the Children’s Hospitals Neonatal Consortium.
References
Abman SH, Collaco JM, Shepherd EG, Keszler M, Cuevas-Guaman M, Welty SE. et al. Interdisciplinary care of Children with Severe Bronchopulmonary Dysplasia. J Pediatr. 2017;181:12–28.e1.
Lagatta JM, Hysinger EB, Zaniletti I, Wymore EM, Vyas-Read S, Yallapragada S, et al. The impact of pulmonary hypertension in preterm infants with severe bronchopulmonary dysplasia through 1 year. J Pediatr. 2018. https://doi.org/10.1016/j.jpeds.2018.07.035.
Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol. 2013;37:124–131.
Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol. 2011;31:635–640.
Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132:2037–2099.
Weismann CG, Asnes JD, Bazzy-Asaad A, Tolomeo C, Ehrenkranz RA, Bizzarro MJ. Pulmonary hypertension in preterm infants: results of a prospective screening program. J Perinatol. 2017;37:572–577.
Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121:317–325.
McCrary AW, Barker PCA, Torok RD, Spears TG, Li JS, Hornik CP, et al. Agreement of an echocardiogram-based diagnosis of pulmonary hypertension in infants at risk for bronchopulmonary dysplasia among masked reviewers. J Perinatol. 2018. https://doi.org/10.1038/s41372-018-0277-6.
Carlton EF, Sontag MK, Younoszai A, DiMaria MV, Miller JI, Poindexter BB, et al. Reliability of echocardiographic indicators of pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. J Pediatr. 2017. https://doi.org/10.1016/j.jpeds.2017.03.027.
Murthy K, Porta NFM, Lagatta JM, Zaniletti I, Truog WE, Grover TR, et al. Inter-center variation in death or tracheostomy placement in infants with severe bronchopulmonary dysplasia. J Perinatol. 2017;37:723–727.
Hysinger EB, Friedman NL, Padula MA, Shinohara RT, Zhang H, Panitch HB, et al. Tracheobronchomalacia is associated with increased morbidity in bronchopulmonary dysplasia. Ann Am Thorac Soc. 2017;14. https://doi.org/10.1513/AnnalsATS.201702-178OC.
Mirza H, Ziegler J, Ford S, Padbury J, Tucker R, Laptook A. Pulmonary hypertension in preterm infants: prevalence and association with bronchopulmonary dysplasia. J Pediatr. 2014;165:909–14.e1.
del Cerro MJ, Sabate Rotes A, Carton A, Deiros L, Bret M, Cordeiro M, et al. Pulmonary hypertension in bronchopulmonary dysplasia: clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol. 2014;49:49–59.
Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–24.
Al-Ghanem G, Shah P, Thomas S, Banfield L, Helou El S, Fusch C. et al. Bronchopulmonary dysplasia and pulmonary hypertension: a meta-analysis. J Perinatol. 2017;37:414–419.
Ali Z, Schmidt P, Dodd J, Jeppesen DL. Predictors of bronchopulmonary dysplasia and pulmonary hypertension in newborn children. Dan Med J. 2013;60:A4688.
Baker CD, Abman SH, Mourani PM. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Pediatr Allergy, Immunol, Pulmonol. 2014;27:8–16.
Murthy K, Savani RC, Lagatta JM, Zaniletti I, Wadhawan R, Truog W, et al. Predicting death or tracheostomy placement in infants with severe bronchopulmonary dysplasia. J Perinatol. 2014;34:543–548.
Abraham S, Weismann CG. Left ventricular end-systolic eccentricity index for assessment of pulmonary hypertension in infants. Echocardiography. 2016;33:910–915.
Zivanovic S, Pushparajah K, Calvert S, Marlow N, Razavi R, Peacock JL. et al. Pulmonary artery pressures in school-age children born prematurely. J Pediatr. 2017;191:42–49.e3.
Watson T, McCracken CE, Slesnick T, Kanaan U, Border WL, Sachdeva R. Quantitative assessment of ventricular septal contour for estimation of right ventricular pressure. Echocardiography. 2016;33:444–9. quiz 443
McCrary AW, Malowitz JR, Hornick CP, Hill KD, Cotten CM, Tatum GH, et al. Differences in eccentricity index and systolic-diastolic ratio in extremely low-birth-weight infants with bronchopulmonary dysplasia at risk of pulmonary hypertension. Am J Perinatol. 2016;33:57–62.
Altit G, Lee HC, Hintz S, Tacy TA, Feinstein JA, Bhombal S. Practices surrounding pulmonary hypertension and bronchopulmonary dysplasia amongst neonatologists caring for premature infants. J Perinatol. 2018;38:361–367.
Mehler K, Udink Ten Cate FE, Keller T, Bangen U, Kribs A, Oberthuer A. An echocardiographic screening program helps to identify pulmonary hypertension in extremely low birthweight infants with and without bronchopulmonary dysplasia: a single-center experience. Neonatology. 2018;113:81–88.
An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010;40:131–136.
Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–1269.
Martin RJ, Di Fiore JM, Walsh MC. Hypoxic episodes in bronchopulmonary dysplasia. Clin Perinatol. 2015;42:825–838.
Kovesi T, Abdurahman A, Blayney M. Elevated carbon dioxide tension as a predictor of subsequent adverse events in infants with bronchopulmonary dysplasia. Lung. 2006;184:7–13.
Thome UH, Dreyhaupt J, Genzel-Boroviczeny O, Bohnhorst B, Schmid M, Fuchs H, et al. Influence of PCO2 control on clinical and neurodevelopmental outcomes of extremely low birth weight infants. Neonatology. 2018;113:221–230.
Brown MK, Poeltler DM, Hassen KO, Lazarus DV, Brown VK, Stout JJ, et al. Incidence of hypocapnia, hypercapnia, and acidosis and the associated risk of adverse events in preterm neonates. Respir Care. 2018;63:943–949.
Krishnan U, Feinstein JA, Adatia I, Austin ED, Mullen MP, Hopper RK, et al. Evaluation and management of pulmonary hypertension in children with bronchopulmonary dysplasia. J Pediatr. 2017. https://doi.org/10.1016/j.jpeds.2017.05.029.
Funding
The Children’s Hospitals Neonatal Consortium (501-c3 organization) supported the statistical analyses presented in this manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
SV-R, EMW, KM, JML: Research question and design, data analysis and interpretation, draft preparation, editing, and revision of the manuscript. IZ: Statistical analysis of the manuscript. MAP, WET, WAE, RCS, SY, JWL, HZ, EBH, TRG, GN, LDN, NFMP, KPP, RD: Data interpretation, editing and revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vyas-Read, S., Wymore, E.M., Zaniletti, I. et al. Utility of echocardiography in predicting mortality in infants with severe bronchopulmonary dysplasia. J Perinatol 40, 149–156 (2020). https://doi.org/10.1038/s41372-019-0508-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0508-5