Abstract
Objective
Describe the characteristics of infants with NAS and determine if treatment outcomes varied between three protocols.
Study design
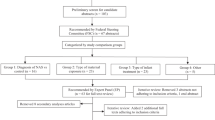
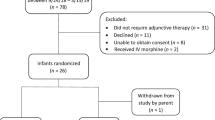
Based on medical record data, infant treatment for NAS-related withdrawal reflected one of three protocols: (1) No rescue dose (n = 836, 52.7%): Prescriber ordered initiation and escalation doses and determined when infants were eligible for weaning, (2) Rescue dose (n = 233, 14.7%): No rescue dose with the addition of a prescriber-ordered rescue dose, (3) Rescue dose by order set (n = 516, 32.6%): Rescue dose with addition of nurse-assisted order of morphine during escalation.
Results
The no rescue dose group had longer length of stay, days to wean, and inpatient days, and greater initial morphine dose than the two rescue dose groups (p < 0.001). Treatment outcomes between the two rescue dose protocols did not differ.
Conclusions
The benefits related to rescue dosing further inform the development of a standardized NAS treatment protocol.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among medicaid-enrolled women. Obstet Gynecol. 2014;123:997–1002.
Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015–2016. Morb Mortal Wkly Report. 2018;67:349–58.
Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: variation among States in prescribing of opioid pain relievers and benzodiazepines - United States, 2012. Morb Mortal Wkly Report. 2014;63:563–8.
Whiteman VE, Salemi JL, Mogos MF, Cain MA, Aliyu MH, Salihu HM. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. J Pregnancy. 2014;2014:906723.
Creanga AA, Sabel JC, Ko JY, Wasserman CR, Shapiro-Mendoza CK, Taylor P, et al. Maternal drug use and its effect on neonates: a population-based study in Washington State. Obstet Gynecol. 2012;119:924–33.
Tolia VN, Patrick SW, Bennett MM, Murthy K, Sousa J, Smith PB, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. New Engl J Med. 2015;372:2118–26.
Ko J, Patrick S, Tong V, Patel R, Lind J, Barfield W. Incidence of Neonatal Abstinence Syndrome — 28 States, 1999–2013. Morb Mortal Wkly Report. 2016;65:799–802.
Patrick SW, Davis MM, Lehman CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35:667.
Patrick SW, Kaplan HC, Passarella M, Davis MM, Lorch SA. Variation in treatment of neonatal abstinence syndrome in US children’s hospitals, 2004-2011. J Perinatol. 2014;34:867–72.
Walsh MC, Crowley M, Wexelblatt S, Ford S, Kuhnell P, Kaplan HC, et al. Ohio perinatal quality collaborative improves care of neonatal narcotic abstinence syndrome. Pediatrics. 2018;141:e20170900.
Behnke M, Smith VC. Committee on Substance A, Committee on F, Newborn. Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009–24.
Finnegan L, Connaughton JJ, Kron R, Emich J. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2:141–58.
Raffaeli G, Cavallaro G, Allegaert K, Wildschut ED, Fumagalli M, Agosti M, et al. Neonatal abstinence syndrome: update on diagnostic and therapeutic strategies. Pharmacotherapy. 2017;37:814–23.
Patrick SW, Schumacher RE, Horbar JD, Buus-Frank ME, Edwards EM, Morrow KA, et al. Improving care for neonatal abstinence syndrome. Pediatrics. 2016;137:e20153835.
Kraft WK, Stover MW, Davis JM. Neonatal abstinence syndrome: pharmacologic strategies for the mother and infant. Semin Perinatol. 2016;40:203–12.
Saunders C, King T, Smith S, Buchheit J, Cook K, Edds J, et al. Neonatal abstinence syndrome: evaluating the effectiveness of an evidence-based multidisciplinary care approach. J Perinat Neonatal Nurs. 2014;28:232–40.
Berens RJ, Meyer MT, Mikhailov TA, Colpaert KD, Czarnecki ML, Ghanayem NS, et al. A prospective evaluation of opioid weaning in opioid-dependent pediatric critical care patients. Anesth Analg. 2006;102:1045–50.
McQueen K, Murphy-Oikonen J. Neonatal Abstinence Syndrome. New Engl J Med. 2016;375:2468–79.
Asti L, Magers JS, Keels E, Wispe J, McClead RE Jr. A quality improvement project to reduce length of stay for neonatal abstinence syndrome. Pediatrics. 2015;135:e1494–500.
D’Apolito K, Donaghey B, Dietrich M, Engelhardt B, Saunders C, Smith S, et al. NAS Management Trends in TN Study Report; 2015. pp. 1–46. https://www.tn.gov/content/dam/tn/health/healthprofboards/2015_DApolito_NAS_Management_Trends.pdf.
Erwin P, Lindley L, Meschke L, Ehrlich S. Neonatal abstinence syndrome in East Tennessee: characteristics and risk factors among mothers and infants in one area of Appalachia. J Health Care Poor Under 2017;28:1393–408.
Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35:650–5.
Miller AM, Warren MD. Neonatal Abstinence Syndrome Surveillance Annual Report 2015. In: Tennessee Department of Health, editor. Nashville, TN2016.
Miller AM, Warren MD. Neonatal Abstinence Syndrome Surveillance Annual Report 2014. In: Tennessee Department of Health, editor. Nashville, TN2015.
Tenneseee Department of Health. NAS Annual Report. 2013.
Tennessee General Assembly. Safe Harbor Act of 2013. 2013.
Melton C, Pellegrin M. The Opioid Epidemic in Tennessee. The Sycamore Institute; 2017. https://www.sycamoreinstitutetn.org/2018/08/09/opioid-epidemic-tn-indicators/.
Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2016. National Vital Statistics Reports. Vol. 67, no. 1. Hyattsville, MD: National Center for Health Statistics; 2018.
Lind JN, Petersen EE, Lederer PA, Phillips-Bell GS, Perrine CG, Li R. Infant and maternal characteristics in neonatal abstinence syndrome—selected hospitals in Florida, 2010–2011. MMWR Morb Mortal Wkly Rep. 2015;64:213–6.
Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. J Am Med Assoc. 2012;307:1934–40.
Ferre C, Callaghan W, Olson C, Sharma A, Barfield W. Effects of maternal age and age-specific preterm birth rates on overall preterm birth rates - United States, 2007 and 2014. Morb Mortal Wkly Report. 2016;65:1181–4.
Tennessee Department of Health. Prevalence of Tobacco Use in Tennessee. 2008.
Dryden C, Young D, Hepburn M, Mactier H. Maternal methadone use in pregnancy: factors associated with the development of neonatal abstinence syndrome and implications for healthcare resources. Int J Obstet Gynaecol. 2009;116:665–71.
Cleary BJ, Eogan M, O’Connell MP, Fahey T, Gallagher PJ, Clarke T, et al. Methadone and perinatal outcomes: a prospective cohort study. Addiction. 2012;107:1482–92.
Cleary BJ, Donnelly JM, Strawbridge JD, Gallagher PJ, Fahey T, White MJ. et al. Methadone and perinatal outcomes: a retrospective cohort study. Am J Obstet Gynecol. 2011;204:139.e1–9.
Doberczak TM, Kandall SR, Wilets I. Neonatal opiate abstinence syndrome in term and preterm infants. J Pediatr. 1991;118:933–7.
Dysart K, Hsieh HC, Kaltenbach K, Greenspan JS. Sequela of preterm versus term infants born to mothers on a methadone maintenance program: differential course of neonatal abstinence syndrome. J Perinat Med. 2007;35:344–6.
Bratton M. Prescription Drug Abuse Report. Nashville, TN: Tennessee Department of Health; 2018.
Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the united states: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hartgrove, M.J., Meschke, L.L., King, T.L. et al. Treating infants with neonatal abstinence syndrome: an examination of three protocols. J Perinatol 39, 1377–1383 (2019). https://doi.org/10.1038/s41372-019-0450-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0450-6
This article is cited by
-
Predictors of pharmacologic therapy for neonatal opioid withdrawal syndrome: a retrospective analysis of a statewide database
Journal of Perinatology (2021)
-
Adverse neonatal outcomes associated with maternal severe mental health diagnoses and opioid use
Journal of Perinatology (2020)