Abstract
Objective
To evaluate the effectiveness of drugs used to constrict patent ductus arteriosus (PDA) in newborns < 28 weeks.
Methods
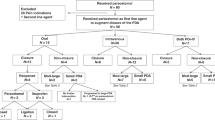
We performed a secondary analysis of the multi-center PDA-TOLERATE trial (NCT01958320). Infants with moderate-to-large PDAs were randomized 1:1 at 8.1 ± 2.1 days to either Drug treatment (n = 104) or Conservative management (n = 98). Drug treatments were assigned by center rather than within center (acetaminophen: 5 centers, 27 infants; ibuprofen: 7 centers, 38 infants; indomethacin: 7 centers, 39 infants).
Results
Indomethacin produced the greatest constriction (compared with spontaneous constriction during Conservative management): RR (95% CI) = 3.21 (2.05–5.01)), followed by ibuprofen = 2.03 (1.05–3.91), and acetaminophen = 1.33 (0.55–3.24). The initial rate of acetaminophen-induced constriction was 27%. Infants with persistent moderate-to-large PDA after acetaminophen were treated with indomethacin. The final rate of constriction after acetaminophen ± indomethacin was 60% (similar to the rate in infants receiving indomethacin-alone (62%)).
Conclusion
Indomethacin was more effective than acetaminophen in producing ductus constriction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
After de-identification individual participant data that underlie the results reported in this article will be available to researchers who provide a methodologically sound proposal. Proposals should be directed to clymanr@ucsf.edu. To gain access, data requestors will need to sign a data access agreement.
References
Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics. 2006;117:1113–21.
Nemerofsky SL, Parravicini E, Bateman D, Kleinman C, Polin RA, Lorenz JM. The ductus arteriosus rarely requires treatment in infants > 1000 grams. Am J Perinatol. 2008;25:661–6.
Sung SI, Chang YS, Chun JY, Yoon SA, Yoo HS, Ahn SY, et al. Mandatory closure versus nonintervention for patent ductus arteriosus in very preterm infants. J Pediatr. 2016;177:66–71.
Hammerman C, Bin-Nun A, Markovitch E, Schimmel MS, Kaplan M, Fink D. Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics. 2011;128:e1618–21.
Allegaert KS, Anderson B, Simons S, Van Overmeire B. Paracetamol to induce ductus arteriosus closure: is it valid? Arch Dis Child. 2013;98:462–6.
El-Khuffash A, Jain A, Corcoran D, Shah PS, Hooper CW, Brown N, et al. Efficacy of paracetamol on patent ductus arteriosus closure may be dose dependent: evidence from human and murine studies. Pediatr Res. 2014;76:238–44.
Al-Lawama M, Alammori I, Abdelghani T, Badran E. Oral paracetamol versus oral ibuprofen for treatment of patent ductus arteriosus. J Int Med Res. 2018;46:811–8.
Dang D, Wang D, Zhang C, Zhou W, Zhou Q, Wu H. Comparison of oral paracetamol versus ibuprofen in premature infants with patent ductus arteriosus: a randomized controlled trial. PLoS ONE. 2013;8:e77888.
Dash SK, Kabra NS, Avasthi BS, Sharma SR, Padhi P, Ahmed J. Enteral paracetamol or Intravenous Indomethacin for closure of patent ductus arteriosus in preterm neonates: a randomized controlled trial. Indian Pediatr. 2015;52:573–8.
El-Mashad AE, El-Mahdy H, El Amrousy D, Elgendy M. Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. Eur J Pediatr. 2017;176:233–40.
Harkin P, Harma A, Aikio O, Valkama M, Leskinen M, Saarela T, et al. Paracetamol accelerates closure of the ductus arteriosus after premature birth: a randomized trial. J Pediatr. 2016;177:72–7.
Oncel MY, Yurttutan S, Erdeve O, Uras N, Altug N, Oguz SS, et al. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Pediatr. 2014;164:510–4.
Yang B, Gao X, Ren Y, Wang Y, Zhang Q. Oral paracetamol vs. oral ibuprofen in the treatment of symptomatic patent ductus arteriosus in premature infants: A randomized controlled trial. Exp Ther Med. 2016;12:2531–6.
Bagheri MM, Niknafs P, Sabsevari F, Torabi MH, Bahman Bijari B, Noroozi E, et al. Comparison of Oral Acetaminophen Versus Ibuprofen in Premature Infants With Patent Ductus Arteriosus. Iran J Pediatr. 2016;26:e3975.
El-Farrash RA, El Shimy MS, El-Sakka AS, Ahmed MG, Abdel-Moez DG. Efficacy and safety of oral paracetamol versus oral ibuprofen for closure of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1–8. https://doi.org/10.1080/14767058.2018.1470235. [Epub ahead of print]
Asbagh PA, Zarkesh MR, Nili F, Sadat Nayeri FS, Naeem AT. Prophylactic treatment with oral paracetamol for patent ductus arteriosus in preterm infants: a randomized clinical trial. Tehran Univ Med J. 2015;73:86–92.
Terrin G, Conte F, Oncel MY, Scipione A, McNamara PJ, Simons S, et al. Paracetamol for the treatment of patent ductus arteriosus in preterm neonates: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2016;101:F127–36.
Ohlsson A, Shah PS. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst Rev. 2018;4:CD010061.
Mitra S, Florez ID, Tamayo ME, Mbuagbaw L, Vanniyasingam T, Veroniki AA, et al. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and Meta-analysis. JAMA. 2018;319:1221–38.
Clyman RI, Liebowitz M, Kaempf J, Erdeve O, Bulbul A, Hakansson S, et al. PDA-TOLERATE trial: an exploratory randomized controlled trial of treatment of moderate-to-large patent ductus arteriosus at one week of age. J Pediatr. 2018;205:41–7.
Schena F, Francescato G, Cappelleri A, Picciolli I, Mayer A, Mosca F, et al. Association between hemodynamically significant patent ductus arteriosus and bronchopulmonary dysplasia. J Pediatr. 2015;166:1488–92.
Sellmer A, Bjerre JV, Schmidt MR, McNamara PJ, Hjortdal VE, Host B, et al. Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch Dis Child Fetal Neonatal Ed. 2013;98:F505–10.
Jhaveri N, Moon-Grady A, Clyman RI. Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. J Pediatr. 2010;157:381–7.
El Hajjar M, Vaksmann G, Rakza T, Kongolo G, Storme L. Severity of the ductal shunt: a comparison of different markers. Arch Dis Child Fetal Neonatal Ed. 2005;90:F419–22.
Hochwald O, Mainzer G, Borenstein-Levin L, Jubran H, Dinur G, Zucker M, et al. Adding paracetamol to ibuprofen for the treatment of patent ductus arteriosus in preterm infants: a double-blind, randomized, placebo-controlled pilot study. Am J Perinatol. 2018. https://doi.org/10.1055/s-0038-1653946.
Furzan JA, Reisch J, Tyson JE, Laird P, Rosenfeld CR. Incidence and risk factors for symptomatic patent ductus arteriosus among inborn very-low-birth-weight infants. Early Hum Dev. 1985;12:39–48.
Clyman RI, Ballard PL, Sniderman S, Ballard RA, Roth R, Heymann MA, et al. Prenatal administration of betamethasone for prevention of patient ductus arteriosus. J Pediatr. 1981;98:123–6.
Chorne N, Jegatheesan P, Lin E, Shi R, Clyman RI. Risk factors for persistent ductus arteriosus patency during indomethacin treatment. J Pediatr. 2007;151:629–34.
Durrmeyer X, Hovhannisyan S, Medard Y, Jacqz-Aigrain E, Decobert F, Barre J, et al. Are cytochrome P450 CYP2C8 and CYP2C9 polymorphisms associated with ibuprofen response in very preterm infants? PLoS ONE. 2010;5:e12329.
Cotton RB, Haywood JL, FitzGerald GA. Symptomatic patent ductus arteriosus following prophylactic indomethacin. A clinical and biochemical appraisal. Biol Neonate. 1991;60:273–82.
Waleh N, Barrette AM, Dagle JM, Momany A, Jin C, Hills NK, et al. Effects of advancing gestation and non-caucasian race on ductus arteriosus gene expression. J Pediatr. 2015;167:1033–41.
Shelton EL, Waleh N, Plosa EJ, Benjamin JT, Milne GL, Hooper CW, et al. Effects of antenatal betamethasone on preterm human and mouse ductus arteriosus: comparison with baboon data. Pediatr Res. 2018. https://doi.org/10.1038/s41390-018-0006-z.
Momma K, Takao A. Transplacental cardiovascular effects of four popular analgesics in rats. Am J Obstet Gynecol. 1990;162:1304–10.
Momma K, Hagiwara H, Konishi T. Constriction of fetal ductus arteriosus by non-steroidal anti-inflammatory drugs: study of additional 34 drugs. Prostaglandins. 1984;28:527–36.
Sancak S, Gokmen Yildirim T, Topcuoglu S, Yavuz T, Karatekin G, Ovali F. Oral versus intravenous paracetamol: which is better in closure of patent ductus arteriosus in very low birth weight infants? J Matern Fetal Neonatal Med. 2016;29:135–9.
Bin-Nun A, Fink D, Mimouni FB, Algur N, Hammerman C. Paracetamol serum concentrations in neonates treated enterally for ductal closure: a pilot study. J Pediatr. 2018;198:304–7.
Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010:CD000174. https://doi.org/10.1002/14651858.CD000174.pub2.
Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2011:CD004213. https://doi.org/10.1002/14651858.CD004213.pub3.
van den Anker JN, Allegaert K. Acetaminophen in the neonatal intensive care unit: shotgun approach or silver bullet. J Pediatr. 2018;198:10–1.
Acknowledgements
We would like to thank the following PDA-TOLERATE investigators without whom this study would not have been possible: University of California San Francisco, San Francisco, CA: Scott Fields, PharmD; Providence St. Vincent Medical Center, Portland, OR: Lora Whitten, RN, Stefanie Rogers, MD; Ankara University School of Medicine Children’s Hospital, Ankara, Turkey: Emel Okulu, MD, Begum Atasay, MD, Saadet Arsan, MD; Sisli Hamidiye Etfal Training and Research Hospital, İstanbul, Turkey: Ebru Türkoglu Ünal, MD; Sharp Mary Birch Hospital, San Diego, CA: Jane Steen, RN, Kathy Arnell, RN; University of Chicago, Chicago, IL: Sarah Holtschlag, RN, Michael Schreiber, MD; Morristown Medical Center, Morristown, NJ: Caryn Peters, RN; Johns Hopkins Hospital, Baltimore, MD: Maureen Gilmore, MD; University of Glasgow, Royal Hospital for Sick Children, Glasgow, Scotland, UK: Lorna McKay, RN, Dianne Carole, RN, Annette Shaw, RN; Mayo Clinic, Rochester, MN: Malinda Harris, MD, Amy Amsbaugh, RRT, Lavonne M. Liedl, RRT; Northshore University Health System, Evanston, IL: Avi Groner, MD; University of California San Diego and Rady Children’s Hospital, San Diego, CA: Erika Fernandez, MD, Jae Kim, MD, Renee Bridge, RN, Ellen Knodel, RN; Good Samaritan Hospital, San Jose, CA: Chrissy Weng, RN; South Miami Hospital/Baptist Health South Florida, Miami, FL: Magaly Diaz Barbosa, MD; Columbia University Medical Center, New York, NY: Richard Polin, MD, Marilyn Weindler, RN; Data Safety Monitoring Committee: Shahab Noori, MD, University of Southern California, Los Angeles, CA, Jeffrey Reese, MD, Vanderbilt University, Nashville, TN, Yao Sun, MD, University of California San Francisco, San Francisco, CA. We also would like to thank Dr. Mark Cocalis and the cardiologists at all of the participating institutions for their expert help in reading the echocardiograms. This work was supported by grants from the Gerber Foundation, U.S. Public Health Service National Heart, Lung and Blood Institute (HL109199), National Center for Advancing Translational Sciences, National Institutes of Health, through (UL1 TR001872, UL1 TR000004 and UL1TR001873), and a gift from the Jamie and Bobby Gates Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liebowitz, M., Kaempf, J., Erdeve, O. et al. Comparative effectiveness of drugs used to constrict the patent ductus arteriosus: a secondary analysis of the PDA-TOLERATE trial (NCT01958320). J Perinatol 39, 599–607 (2019). https://doi.org/10.1038/s41372-019-0347-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0347-4
This article is cited by
-
Acetaminophen for the patent ductus arteriosus: has safety been adequately demonstrated?
Journal of Perinatology (2023)
-
Dual medication therapy (acetaminophen and ibuprofen) for the management of patent ductus arteriosus in preterm infants: a systematic review and meta-analysis
Journal of Perinatology (2022)
-
Oral versus intravenous paracetamol for patent ductus arteriosus closure in preterm infants
Pediatric Research (2022)
-
Efficacy and Costs of Three Pharmacotherapies for Patent Ductus Arteriosus Closure in Premature Infants
Pediatric Drugs (2022)
-
A randomized trial of intravenous acetaminophen versus indomethacin for treatment of hemodynamically significant PDAs in VLBW infants
Journal of Perinatology (2021)