Abstract
Objective
Non-invasive prenatal screening (NIPS) utilizes circulating cell-free DNA (cfDNA) to screen for fetal genetic abnormalities. NIPS is the first widely-available prenatal screen to assess genotypic sex. Most pediatricians have limited familiarity with NIPS technology and potential etiologies of discordant results. Increased familiarity may provide diagnostic insight and improve clinical care.
Study design
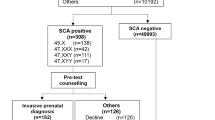
We reviewed all patients with discordant genotypic fetal sex assessed by cfDNA and neonatal phenotypic sex referred to our medical center.
Result
Four infants with discordant cfDNA result and phenotypic sex were identified. Etiologies include vanishing twin syndrome, difference of sexual development, sex chromosome aneuploidy and maternal chimerism.
Conclusions
We present four cases illustrating potential etiologies of discordant cfDNA result and postnatal phenotypic sex. Unanticipated cfDNA results offer the perinatologist a unique opportunity for early diagnosis and targeted treatment of various conditions, many of which may not have otherwise been detected in the perinatal period.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bianchi DW, Parsa S, Bhatt S, Halks-Miller M, Kurtzman K, Sehnert AJ, et al. Fetal sex chromosome testing by maternal plasma DNA sequencing: clinical laboratory experience and biology. Obstet Gynecol. 2015;125:375–82.
Wapner RJ, Babiarz JE, Levy B, Stosic M, Zimmermann B, Sigurjonsson S, et al. Expanding the scope of noninvasive prenatal testing: detection of fetal microdeletion syndromes. Am J Obstet Gynecol. 2015;212:332.e1–9.
Neufeld-Kaiser WA, Cheng EY, Liu YJ. Positive predictive value of non-invasive prenatal screening for fetal chromosome disorders using cell-free DNA in maternal serum: independent clinical experience of a tertiary referral center. BMC Med. 2015;13:129.
Snyder MW, Simmons LE, Kitzman JO, Coe BP, Henson JM, Daza RM, et al. Copy- number variation and false positive prenatal aneuploidy screening results. N Engl J Med. 2015;372:1639–45.
Gil MM, Quezada MS, Revello R, Akolekar R, Nicolaides KH. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2015;45:249–66.
Devaney SA, Palomaki GE, Scott JA, Bianchi DW. Noninvasive fetal sex determination using cell-free fetal DNA: a systematic review and meta-analysis. JAMA. 2011;306:627–36.
Daley R, Hill M, Chitty LS. Non-invasive prenatal diagnosis: progress and potential. Arch Dis Child Fetal Neonatal Ed. 2014;99:F426–30.
Richardson EJ, Scott FP, McLennan AC. Sex discordance identification following non- invasive prenatal testing. Prenat Diagn. 2017;37:1298–304.
Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–7.
Colmant C, Morin-Surroca M, Fuchs F, Fernandez H, Senat MV. Non-invasive prenatal testing for fetal sex determination: is ultrasound still relevant? Eur J Obstet Gynecol Reprod Biol. 2013;171:197–204.
Gil MM, Accurti V, Santacruz B, Plana MN, Nicolaides KH. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2017;50:302–14.
American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med. 2013;32:1083–101.
Shin SY, Yoo HW, Lee BH, Kim KS, Seo EJ. Identification of the mechanism underlying a human chimera by SNP array analysis. Am J Med Genet. 2012;158:2119–23.
Masala M, Saba L, Zoppi MA, Puddu R, Picciau A, Capponi V, et al. Pitfalls in noninvasive fetal RhD and sex determination due to a vanishing twin. Prenat Diagn. 2015;35:506–8.
Curnow KJ, Wilkins-Haug L, Ryan A, Kırkızlar E, Stosic M, Hall MP, et al. Detection of triploid, molar, and vanishing twin pregnancies by a single-nucleotide polymorphism- based noninvasive prenatal test. Am J Obstet Gynecol. 2015;212:e1–9.
Chitty LS, Chatelain P, Wolffenbuttel KP, Aigrain Y. Prenatal management of disorders of sex development. J Pediatr Urol. 2012;8:576–84.
Baetens D, Mladenov W, Delle Chiaie B, Menten B, Desloovere A, Iotova V, et al. Extensive clinical, hormonal, and genetic screening in a large consecutive series of 46,XY neonates and infants with atypical sexual development. Orphanet J Rare Dis. 2014;9:209.
Franasiak JM, Yao X, Ashkinadze E, Rosen T, Scott RT Jr. Discordant embryonic aneuploidy testing and prenatal ultrasonography prompting androgen insensitivity syndrome diagnosis. Obstet Gynecol. 2015;125:383-6.
Mansfield N, Boogert T, McLennan A. Prenatal diagnosis of a 46,XX male following noninvasive prenatal testing. Clin Case Rep. 2015;3:849–53.
Parisi MA, Ramsdell LA, Burns MW, Carr MC, Grady RE, Gunther DF, et al. A Gender Assessment Team: experience with 250 patients over a period of 25 years. Genet Med. 2007;9:348–57.
Coyle D, Kutasy B, Han Suyin K, Antao B, Lynch SA, McDermott MB, et al. Gonadoblastoma in patients with 45,X/46,XY mosaicism: A 16-year experience. J Pediatr Urol. 2016;12:283.e1–283.e7.
Tosson H, Rose SR, Gartner LA. Description of children with 45,X/46,XY karyotype. Eur J Pediatr. 2012;171:521–529.2.
Xu J, Siu VM. Is there a correlation between the proportion of cells with isodicentric Yp at amniocentesis and phenotypic sex? Prenat Diagn. 2010;3:839–44.
Iruretagoyena JI, Grady M, Shah D. Discrepancy in fetal sex assignment between cell free fetal DNA and ultrasound. J Perinatol. 2015;35:229–30.
Colindres JV, Axelrad M, McCullough L, Smith EO, Huang GO, Tu DD, et al. Evidence- Based Management of Patients with 45,X/46,XY Gonadal Dysgenesis and Male Sex Assignment: from Infancy to Adulthood. Pediatr Endocrinol Rev. 2016;13:585–601.
Snyder TM, Khush KK, Valantine HA, Quake SR. Universal noninvasive detection of solid organ transplant rejection. Proc Natl Acad Sci USA. 2011;108:6229–34.
Souter VL, Parisi MA, Nyholt DR, Kapur RP, Henders AK, Opheim KE, et al. A case of true hermaphroditism reveals an unusual mechanism of twinning. Hum Genet. 2007;121:179–85.
de Andrade Machado Neto F, Moreno Morcillo A, Trevas Maciel-Guerra A, Guerra- Junior G. Idiopathic male pseudohermaphroditism is associated with prenatal growth retardation. Eur J Pediatr. 2005;164:287–91.
Lek N, Miles H, Bunch T, Pilfold-Wilkie V, Tadokoro-Cuccaro R, Davies J, et al. Low frequency of androgen receptor gene mutations in 46 XY DSD, and fetal growth restriction. Arch Dis Child. 2014;99:358–61.
Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr. 2004;134:2169–72.
Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–8.
Acknowledgements
Thank you to Lauren H. Brown, MS; Sheila Weiss, MS; Patricia Fechner, MD; Linda Ramsdell, MS; Margaret Shnorhavorian, MD; Elizabeth McCauley, PhD; Anne-Marie Amies-Oelschlager, MD, and Jane Hitti, MD for their contributions to the patient’s clinical care, enthusiasm for this case series and interesting discussions. Funding support provided by the National Institutes of Health NIH5T32GM007454 (H.M.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Byers, H.M., Neufeld-Kaiser, W., Chang, E.Y. et al. Discordant sex between fetal screening and postnatal phenotype requires evaluation. J Perinatol 39, 28–33 (2019). https://doi.org/10.1038/s41372-018-0278-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-018-0278-5
This article is cited by
-
Towards improved genetic diagnosis of human differences of sex development
Nature Reviews Genetics (2021)