Abstract
Objective
Extremely premature infants are at risk for childhood wheezing. Early respiratory support and intermittent hypoxemia (IH) events may be associated with adverse breathing outcomes.
Study design
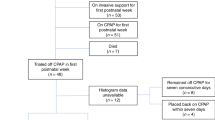
A single-center retrospective cohort study of 137 premature infants <28 weeks gestational age characterized the associations of cumulative oxygen, cumulative mean airway pressure, IH, and oxygen saturation (SpO2) on the primary outcome of prescription asthma medication use at 2-year follow-up. Relative risk was calculated by generalized estimating equations.
Results
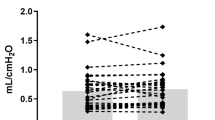
Reported asthma medication use was 46%. At 1–3 days of age, elevated cumulative oxygen exposure, increased daily IH, and lower mean SpO2 (adjusted for gestational age and sex) and increased cumulative mean airway pressure exposure (unadjusted) were associated with asthma medication use.
Conclusion
Increased oxygen and frequent IH events during just the first 3 days of age may help identify extremely premature newborns at risk for symptomatic childhood wheezing requiring prescription asthma medications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital signs: asthma in children - United States, 2001-2016. MMWR Morb Mortal Wkly Rep. 2018;67:149–55.
Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001596.
Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823–30.
Grischkan J, Storfer-Isser A, Rosen CL, Larkin EK, Kirchner HL, South A, et al. Variation in childhood asthma among former preterm infants. J Pediatr. 2004;144:321–6.
Edwards MO, Kotecha SJ, Lowe J, Richards L, Watkins WJ, Kotecha S. Management of prematurity-associated wheeze and its association with atopy. PLoS ONE. 2016;11:e0155695.
Doyle LW, Carse E, Adams AM, Ranganathan S, Opie G, Cheong JLY, et al. Ventilation in extremely preterm infants and respiratory function at 8 years. N Engl J Med. 2017;377:329–37.
Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010;182:237–45.
Di Fiore JM, Martin RJ, Gauda EB. Apnea of prematurity--perfect storm. Respir Physiol Neurobiol. 2013;189:213–22.
Bancalari E, Claure N. Respiratory instability and hypoxemia episodes in preterm infants. Am J Perinatol. 2018;35:534–6.
Di Fiore JM, Walsh M, Wrage L, Rich W, Finer N, Carlo WA, et al. Low oxygen saturation target range is associated with increased incidence of intermittent hypoxemia. J Pediatr. 2012;161:1047–52.
Di Fiore JM, Bloom JN, Orge F, Schutt A, Schluchter M, Cheruvu VK, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 2010;157:69–73.
Abu Jawdeh EG, Martin RJ, Dick TE, Walsh MC, Di Fiore JM. The effect of red blood cell transfusion on intermittent hypoxemia in ELBW infants. J Perinatol. 2014;34:921–5.
Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, Bader D, et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA. 2015;314:595–603.
Fairchild KD, Lake DE. Cross-correlation of heart rate and oxygen saturation in very low birthweight infants: association with apnea and adverse events. Am J Perinatol. 2018;35:463–9.
Di Fiore JM, Martin RJ, Li H, Morris N, Carlo WA, Finer N, et al. Patterns of oxygenation, mortality, and growth status in the Surfactant Positive Pressure and Oxygen Trial cohort. J Pediatr. 2017;186:49–56.e41.
Stevens TP, Dylag A, Panthagani I, Pryhuber G, Halterman J. Effect of cumulative oxygen exposure on respiratory symptoms during infancy among VLBW infants without bronchopulmonary dysplasia. Ped Pulmonol. 2010;45:371–9.
Halvorsen T, Skadberg BT, Eide GE, Roksund O, Aksnes L, Oymar K. Characteristics of asthma and airway hyper-responsiveness after premature birth. Pediatr Allergy Immunol. 2005;16:487–94.
Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100.
Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23:451–6.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
The Stop-ROP Multi-Center Study Group. Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105:295–310.
Wai KC, Kohn MA, Ballard RA, Truog WE, Black DM, Asselin JM, et al. Early cumulative supplemental oxygen predicts bronchopulmonary dysplasia in high risk extremely low gestational age newborns. J Pediatr. 2016;177:97–102 e102.
Wilkinson DJ, Andersen CC, Smith K, Holberton J. Pharyngeal pressure with high-flow nasal cannulae in premature infants. J Perinatol. 2008;28:42–47.
Di Fiore JM, Kaffashi F, Loparo K, Sattar A, Schluchter M, Foglyano R, et al. The relationship between patterns of intermittent hypoxia and retinopathy of prematurity in preterm infants. Pediatr Res. 2012;72:606–12.
Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Andreias L, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. JAMA. 2005;294:318–25.
Hack M, Schluchter M, Andreias L, Margevicius S, Taylor HG, Drotar D, et al. Change in prevalence of chronic conditions between childhood and adolescence among extremely low-birth-weight children. JAMA. 2011;306:394–401.
Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8.
Boggs E, Minich N, Hibbs AM. Performance of commonly used respiratory questionnaire items in a cohort of infants born preterm. Open J Pediatr. 2013;3:260–5.
Stevens TP, Finer NN, Carlo WA, Szilagyi PG, Phelps DL, Walsh MC, et al. Respiratory outcomes of the Surfactant Positive Pressure and Oximetry Randomized Trial (SUPPORT). J Pediatr. 2014;165:240–9.
Kotecha SJ, Edwards MO, Watkins WJ, Henderson AJ, Paranjothy S, Dunstan FD, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax. 2013;68:760–6.
Filippone M, Carraro S, Baraldi E. The term “asthma” should be avoided in describing the chronic pulmonary disease of prematurity. Eur Respir J. 2013;42:1430–1.
Reyburn B, Martin RJ, Prakash YS, MacFarlane PM. Mechanisms of injury to the preterm lung and airway: implications for long-term pulmonary outcome. Neonatology. 2012;101:345–52.
McEvoy CT, Park BS, Spindel ER. Sensitive windows for in utero exposures and asthma development. Layers of complexity. Am J Respir Crit Care Med. 2017;196:1362–4.
Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–55.
Liu J, Li Z, Liu Z, Zhu F, Li W, Jiang H, et al. Exhaled nitric oxide from the central airway and alveoli in OSAHS patients: the potential correlations and clinical implications. Sleep Breath. 2016;20:145–54.
Tichanon P, Wilaiwan K, Sopida S, Orapin P, Watchara B, Banjamas I. Effect of continuous positive airway pressure on airway inflammation and oxidative stress in patients with obstructive sleep apnea. Can Respir J. 2016;2016:3107324.
Cornette L. Fetal and neonatal inflammatory response and adverse outcome. Semin Fetal Neonatal Med. 2004;9:459–70.
Kalikstad B, Kultima HG, Andersstuen TK, Klungland A, Isaksson A. Gene expression profiles in preterm infants on continuous longterm oxygen therapy suggest reduced oxidative stressdependent signaling during hypoxia. Mol Med Rep. 2017;15:1513–26.
Bowser JL, Lee JW, Yuan X, Eltzschig HK. The hypoxia-adenosine link during inflammation. J Appl Physiol (1985). 2017;123:1303–20.
Del Rio R, Moya EA, Iturriaga R. Differential expression of pro-inflammatory cytokines, endothelin-1 and nitric oxide synthases in the rat carotid body exposed to intermittent hypoxia. Brain Res. 2011;1395:74–85.
Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, et al. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci USA. 2009;106:1199–204.
Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–85.
Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2010;174:307–16.
Dylag AM, Mayer CA, Raffay TM, Martin RJ, Jafri A, MacFarlane PM. Long-term effects of recurrent intermittent hypoxia and hyperoxia on respiratory system mechanics in neonatal mice. Pediatr Res. 2017;81:565–71.
Kumar VH, Lakshminrusimha S, Kishkurno S, Paturi BS, Gugino SF, Nielsen L, et al. Neonatal hyperoxia increases airway reactivity and inflammation in adult mice. Pediatr Pulmonol. 2016;51:1131–41.
Raffay TM, Dylag AM, Di Fiore JM, Smith LA, Einisman HJ, Li Y, et al. S-nitrosoglutathione attenuates airway hyperresponsiveness in murine bronchopulmonary dysplasia. Mol Pharmacol. 2016;90:418–26.
Chang M, Bany-Mohammed F, Kenney MC, Beharry KD. Effects of a superoxide dismutase mimetic on biomarkers of lung angiogenesis and alveolarization during hyperoxia with intermittent hypoxia. Am J Transl Res. 2013;5:594–607.
Mankouski A, Kantores C, Wong MJ, Ivanovska J, Jain A, Benner EJ, et al. Intermittent hypoxia during recovery from neonatal hyperoxic lung injury causes long-term impairment of alveolar development: A new rat model of BPD. Am J Physiol Lung Cell Mol Physiol. 2017;312:L208–L216.
Ratner V, Slinko S, Utkina-Sosunova I, Starkov A, Polin RA, Ten VS. Hypoxic stress exacerbates hyperoxia-induced lung injury in a neonatal mouse model of bronchopulmonary dysplasia. Neonatology. 2009;95:299–305.
Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–9.
Acknowledgements
AMD was supported by the NIH T32HD060537 (PI Richard Martin) and TMR by the NIH K08HL133459-01A1. The study was also supported by NIH R01HL056470 (PI Richard Martin). The authors thank Marissa Mancuso and Dominic Camperchiolo for assisting with chart recovery and data entry. AMD and TMR are participants in the NIH Loan Repayment Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Di Fiore, J.M., Dylag, A.M., Honomichl, R.D. et al. Early inspired oxygen and intermittent hypoxemic events in extremely premature infants are associated with asthma medication use at 2 years of age. J Perinatol 39, 203–211 (2019). https://doi.org/10.1038/s41372-018-0264-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-018-0264-y
This article is cited by
-
Maturation of cardioventilatory physiological trajectories in extremely preterm infants
Pediatric Research (2024)
-
Prematurity-associated wheeze: current knowledge and opportunities for further investigation
Pediatric Research (2023)
-
Pulse oximetry reliability for detection of hypoxemia under motion in extremely premature infants
Pediatric Research (2023)
-
Hypoxemia events in preterm neonates are associated with urine oxidative biomarkers
Pediatric Research (2023)
-
Risk of asthma in preterm infants with bronchopulmonary dysplasia: a systematic review and meta-analysis
World Journal of Pediatrics (2023)