Abstract
Objectives:
The objective of this study was to define the association between the burden of severe hypoxemia (SpO2 ≤70%) in the first week of life and development of severe ICH (grade III/IV) in preterm infants.
Study design:
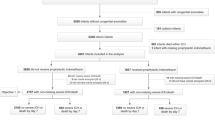
Infants born at <32 weeks or weighing <1500 g underwent prospective SpO2 recording from birth through 7 days. Severe hypoxemia burden was calculated as the percentage of the error-corrected recording where SpO2 ≤70%. Binary logistic regression was used to model the relationship between hypoxemia burden and severe ICH.
Results:
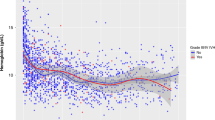
A total of 163.3 million valid SpO2 data points were collected from 645 infants with mean EGA = 27.7 ± 2.6 weeks, BW = 1005 ± 291 g; 38/645 (6%) developed severe ICH. There was a greater mean hypoxemia burden for infants with severe ICH (3%) compared to those without (0.1%) and remained significant when controlling for multiple confounding factors.
Conclusion:
The severe hypoxemia burden in the first week of life is strongly associated with severe ICH.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56.
Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997–1003.
Jain NJ, Kruse LK, Demissie K, Khandelwal M. Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. J Matern Fetal Neonatal Med. 2009;22:491–500.
Ment LR, Duncan CC, Ehrenkranz RA, Lange RC, Taylor KJ, Kleinman CS, et al. Intraventricular hemorrhage in the preterm neonate: timing and cerebral blood flow changes. J Pediatr. 1984;104:419–25.
Ment LR, Ehrenkranz RA, Lange RC, Rothstein PT, Duncan CC. Alterations in cerebral blood flow in preterm infants with intraventricular hemorrhage. Pediatrics. 1981;68:763–9.
Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61:467–73.
Vesoulis ZA, Liao SM, Trivedi SB, Ters NE, Mathur AM. A novel method for assessing cerebral autoregulation in preterm infants using transfer function analysis. Pediatr Res. 2016;79:453–9.
Alderliesten T, Lemmers PMA, Smarius JJM, van de Vosse RE, Baerts W, van Bel F. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J Pediatr. 2013;162:698–704. e2
Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524–51.
Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1–8.
Volpe JJ. Neurology of the newborn. 5th ed. Philadelphia: Saunders/Elsevier; 2008. p. 1094.
Chen J, Smith LEH. Retinopathy of prematurity. Angiogenesis. 2007;10:133–40.
Gogate P, Gilbert C, Zin A. Severe visual Impairment and blindness in infants: causes and opportunities for control. Middle East Afr J Ophthalmol. 2011;18:109.
BOOST II United Kingdom Collaborative Group, BOOST II Australia Collaborative Group, BOOST II New Zealand Collaborative Group, Stenson BJ, Tarnow-Mordi WO, Darlow BA, et al. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368:2094–104.
Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105:295–310.
Sears JE, Pietz J, Sonnie C, Dolcini D, Hoppe G. A change in oxygen supplementation can decrease the incidence of retinopathy of prematurity. Ophthalmology. 2009;116:513–8.
Askie LM, Darlow BA, Davis PG, Finer N, Stenson B, Vento M. et al. Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants. Cochrane Database Syst Rev. 2017;4:CD011190
SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–69.
Hyttel-Sorensen S, Pellicer A, Alderliesten T, Austin T, van Bel F, Benders M, et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ. 2015;350:g7635.
Collins J-A, Rudenski A, Gibson J, Howard L, O’Driscoll R. Relating oxygen partial pressure, saturation and content: the haemoglobin–oxygen dissociation curve. Breathe. 2015;11:194–201.
Madan A. Correlation between the levels of SpO2and PaO2. Lung India Organ Indian Chest Soc. 2017;34:307–8.
Martin RJ, Fanaroff AA, Walsh MC. Fanaroff and Martin’s neonatal-perinatal medicine: diseases of the fetus and infant. Philadelphia: Saunders/Elsevier; 2011.
Jay Goldsmith, Edward Karotkin, Gautham Suresh, Martin Keszler. Assisted ventilation of the neonate. St. Louis: Elsevier/Saunders; 2011.
Bolivar JM, Gerhardt T, Gonzalez A, Hummler H, Claure N, Everett R, et al. Mechanisms for episodes of hypoxemia in preterm infants undergoing mechanical ventilation. J Pediatr. 1995;127:767–73.
Shiao SY, Ou CN. Validation of oxygen saturation monitoring in neonates. Am J Crit Care. 2007;16:168–78.
Sullivan BA, Wallman-Stokes A, Isler J, Sahni R, Moorman JR, Fairchild KD, Lake DE. Early Pulse Oximetry Data Improves Prediction of Death and Adverse Outcomes in a Two-Center Cohort of Very Low Birth Weight Infants. Am J Perinatol. 2018 May 28. https://doi.org/10.1055/s-0038-1654712. [Epub ahead of print] PubMed PMID: 29807371.
Kumar NPD, Praveena B. Mortality profile and timing of death in extremely low birth weight infants from 2013 to 2015 admitted to neonatal intensive care unit, Government General Hospital, Anantapur. Int J Sci Study. 2016;4:114–7.
Barton L, Hodgman JE, Pavlova Z. Causes of death in the extremely low birth weight infant. Pediatrics. 1999;103:446–51.
Sjostedt S, Rooth G. Low oxygen tension in the management of newborn infants. Arch Dis Child. 1957;32:397–400.
Harris AP, Sendak MJ, Chung DC, Richardson CA. Validation of arterial oxygen saturation measurements in utero using pulse oximetry. Am J Perinatol. 1993;10:250–4.
Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, Bader D, et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA. 2015;314:595.
Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, et al. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13:477–85.
Mu D, Jiang X, Sheldon RA, Fox CK, Hamrick SEG, Vexler ZS, et al. Regulation of hypoxia-inducible factor 1alpha and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol Dis. 2003;14:524–34.
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8.
Braun A, Xu H, Hu F, Kocherlakota P, Siegel D, Chander P, et al. Paucity of pericytes in germinal matrix vasculature of premature infants. J Neurosci. 2007;27:12012–24.
Ballabh P, Hu F, Kumarasiri M, Braun A, Nedergaard M. Development of tight junction molecules in blood vessels of germinal matrix, cerebral cortex, and white matter. Pediatr Res. 2005;58:791–8.
El-Khoury N, Braun A, Hu F, Pandey M, Nedergaard M, Lagamma EF, et al. Astrocyte end-feet in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res. 2006;59:673–9.
Acknowledgements
The authors wish to acknowledge the contributions of Ryan Colvin, Amy Distler, Gina Myers (WU), and Marilyn Weindler (CUMC) for their assistance in identifying patient records and data collection.
Funding
This work was supported by the following grants: Washington University Institute of Clinical and Translational Sciences KL2 Training Program (NIH/NCATS KL2 TR000450); The Barnes-Jewish Hospital Foundation and the Washington University Institute of Clinical and Translational Sciences Clinical and Translational Funding Program (NIH/NCATS UL1 TR000448); Washington University in St. Louis Center for Biomedical Informatics, Clinical Investigation Data Exploration Repository (NIH/NCATS UL1 TR000448); and the National Institutes of Health, grant numbers HD072071 and HL133708.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vesoulis, Z.A., Bank, R.L., Lake, D. et al. Early hypoxemia burden is strongly associated with severe intracranial hemorrhage in preterm infants. J Perinatol 39, 48–53 (2019). https://doi.org/10.1038/s41372-018-0236-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-018-0236-2
This article is cited by
-
The impact of red blood cell transfusion in preterm neonates on germinal matrix hemorrhage: incidence and grade with correlation to outcome
Egyptian Pediatric Association Gazette (2023)
-
Improving child health through Big Data and data science
Pediatric Research (2023)
-
Maturation of cardioventilatory physiological trajectories in extremely preterm infants
Pediatric Research (2023)
-
Racial discrepancy in pulse oximeter accuracy in preterm infants
Journal of Perinatology (2022)
-
Vital sign metrics of VLBW infants in three NICUs: implications for predictive algorithms
Pediatric Research (2021)